PMBC Trial Outline
The figure below illustrates the temporal sequence of Pittsburgh Mind-Body Center (PMBC) study procedures. See the table below for a more detailed description of the measures taken during each phase of the study.
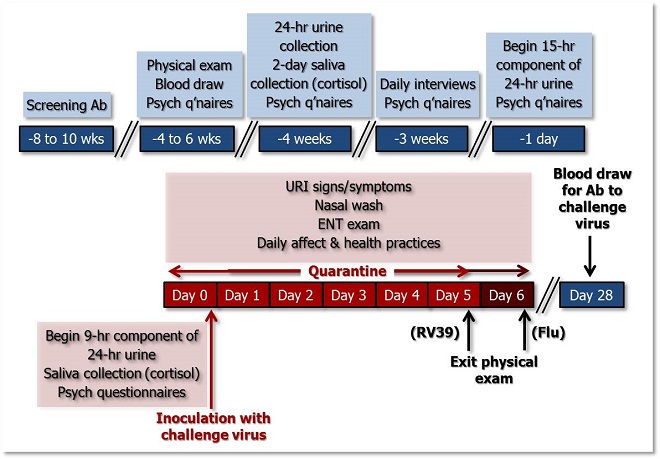
Figure 1. Temporal sequence of PMBC study activities. >>Download PowerPoint Slide<<
PMBC Measures by Study Phase
| Study Phase | Measures Collected |
| 8-10 weeks Pre-Quarantine |
• 10-ml blood draw: viral-specific serum Ab • Screening questionnaires (no data collected) |
| 4 to 6 weeks Pre-Quarantine |
• Screening physical exam • Urine sample: urinalysis; pregnancy test • 25-ml blood draw: o HIV; o Complete blood count (CBC) & serum chemistry; o cytokines (#1) o C-reactive protein (CRP) (#1) • First day of last menstrual period (LMP) (#1) • Questionnaire Set 1 |
| 4 weeks Pre-Quarantine |
• 2-day repeated salivary cortisol collections (#1 & #2) • 24-hour urine collection: catecholamines (#1) • Questionnaire Set 2 |
| 3 weeks Pre-Quarantine |
• Begin 2-week daily interviews o social interactions o affect o health practices • Questionnaire Set 3 • LMP (#2) |
| 1 day Pre-Quarantine |
• Begin 15-hr component of 24-hr urine collection: catecholamines (#2) • Questionnaire Set 4 |
| Quarantine Day 0 (Baseline) |
• Begin 9-hr component of 24-hr urine collection: catecholamines (#2) • Urine sample for pregnancy test • LMP (#3) • 1-day repeated salivary cortisol collection (#3) • Nasal washing for viral isolation • General physical exam • 25-ml blood draw: o cytokines (#2); o CRP (#2); o glucocorticoid resistance • Ear, nose and throat (ENT) exam • Upper respiratory illness (URI) symptoms (self-report) • Daily affect & health practices |
|
End Quarantine Day 0 |
Inoculation with Virus (i.e., Viral Challenge) |
| Quarantine Days 1 though 5* |
• Nasal wash: viral isolation/quantification & cytokines • URI indicators of pathophysiology • General physical examination • ENT exam • URI symptoms (self-report) • Daily affect and health practices |
| End Quarantine Day 5 |
• Exit physical examination |
| 4 weeks Post-Challenge |
• 10-ml blood sample for viral-specific serum Ab • LMP (#4) |
*Participants in the influenza trials (n = 38) were quarantined for 6 rather than 5 days post-challenge.
Study Questionnaire Sets
Questionnaire Set 1
Demographics
MacArthur Scale of Subjective Social Status
Revised Life Orientation Test
Marital-Adjustment Test
Rusbult Accommodation Scale
Social Network Index
Interpersonal Support Evaluation List
Social Convoy Circles
Opener Scale
Pet Ownership Questionnaire
Job Environment Inventory
General Perceived Health
Questionnaire Set 2
Spielberger State-Trait Anger Expression Inventory (#1)
Goldberg's Adjective Scale (Extraversion & Agreeableness subscales only)
Cook-Medley Hostility Scale (#1)
Marital Interpersonal Support Evaluation List
Clark Marital Intimacy Scale
Questionnaire Set 3
Pearlin Mastery Scale
Rosenberg Self-Esteem Scale
Relationship Closeness Inventory
Life Engagement Test
Alcohol Consumption Questionnaire
History and Current Status of Smoking
Paffenbarger Activity Questionnaire
Pittsburgh Sleep Quality Index
Pittsburgh Enjoyable Activities Test
Questionnaire Set 4
Spielberger State-Trait Anger Expression Inventory (#2)
Communal Orientation
Clark Emotional Expression to Spouse Scale
Cohen Crappy Spouse Scale
Ryff Scales of Psychological Well-Being (Positive Relationships Scale only)
Center for Epidemiological Studies Depression Scale Short Form
Questionnaire Set 5
Jourard Self-Disclosure Questionnaire
Braiker & Kelley Marital Conflict Scale
Negative Interaction Items
State Affect Scale
Satisfaction With Life Scale
Perceived Stress Scale
Questionnaire Set 6
Goldberg's Adjective Scale
Cook-Medley Hostility Scale (#2)
Global Religious Background
Rusbult Commitment Scale
Eckenrode Marital Stress Scale
Nan Lin Community Ties Survey
Breakfast