Salivary Cortisol (PCS2, PMBC, PCS3)
Sample Collection
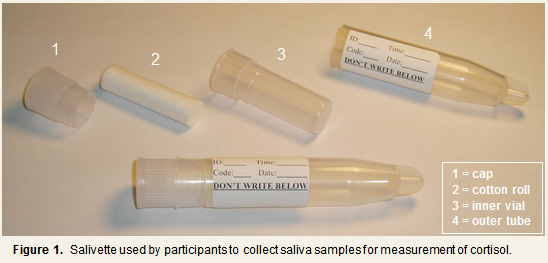
PCS2, PMBC, and PCS3 used salivary measures to assess cortisol level and rhythmicity throughout the waking day. The general procedures for the collection of saliva samples were similar across the three studies, save for the number and timing of saliva collections (see below). Briefly, serial saliva samples were collected from subjects on each of three days: two non-consecutive days occurring 1 to 6 weeks prior to Quarantine and on Quarantine Day 0. Both days of pre-quarantine collections took place in subjects’ natural environments and while engaging in their usual daily activities. Subjects were provided with Salivette ® (Sarstedt, Rommelsdorft, Germany) devices to collect samples of their saliva. The Salivette is comprised of a roll of cotton contained within a specially designed collection tube (see Figure 1). When collecting a sample, subjects placed the cotton roll in their mouths, chewed on it until it became saturated, replaced it into the inner vial of the Salivette, and then tightly capped the outer tube. Subjects were instructed not to eat or brush their teeth for one hour before the scheduled saliva collection time, and to abstain from smoking for 30 minutes before collection time.
Pre-Quarantine Training
Approximately two days prior to their first pre-quarantine assessment day, subjects attended a training session during which they were instructed on how to use a Salivette to collect their saliva samples and when to collect their samples during the two pre-quarantine days. In addition to a set of written instructions and a sufficient number of Salivettes for each pre-quarantine assessment day, a kitchen timer (to be used for collection of the first post-waking sample; see Tables 1 & 2), a personalized saliva collection schedule, and a pre-programmed wristwatch or handheld computer (PalmPilot or WorkPad; only watches were used in PCS2) that signaled subjects at each collection time (see Figure 2). With each signal, the signaling device would display a unique alphanumeric code. A label was affixed to the outside of each Salivette where subjects could record this code in addition to the date and exact time at which the sample was collected. Personalized collection schedules were created and signaling devices programmed based on subjects’ reports of their expected waking time on each of the two pre-quarantine assessment days. Information on wake-up time was obtained at the Follow-up Physical. Subjects in PCS3 also received saliva collection records that they were to complete after collecting the last sample on each pre-quarantine assessment day. Minimal collections were five saliva samples per day for two days.

Quarantine Day 0
The Day 0 sample collection schedules differed somewhat from the pre-quarantine schedules in that the two evening samples were collected on the preceding evening (Day -1) (see Tables 1 & 2). Also, all subjects followed the same schedule rather than a personalized schedule. Procedures for the actual collection of samples on Day 0 were the same as those followed on the 2 pre-quarantine days. The entire set of Day 0 saliva samples was collected from subjects following the last afternoon collection and subjects’ completion of the saliva collection record (PCS3 only).
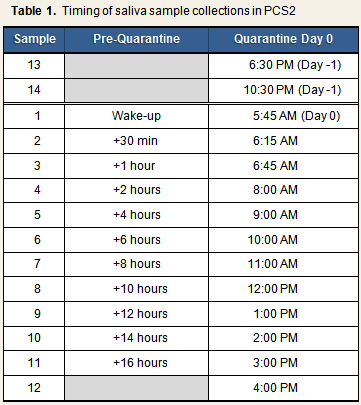
Specific Sample Collection Times: PCS2
On each of the 2 pre-quarantine days, subjects provided 11 saliva samples, with the first sample being collected upon awakening and the last being collected 16 hours after wake-up. During the first 24 hours of quarantine, subjects provided 14 cortisol samples under supervision of the study staff. The first two samples were taken at 6:00 PM and 10:30 PM on Day -1, and the remaining 12 samples were taken on Day 0 (see Table 1).
Specific Sample Collection Times: PMBC & PCS3
On each of the 2 pre-quarantine days, subjects provided 7 saliva samples, with the first sample being collected 1 hour after awakening and the last being collected 11 hours after wake-up. During the first 24 hours of quarantine, subjects provided 8 cortisol samples under supervision of the study staff. The first two samples were taken at 5:00 PM and 10:00 PM on Day -1, and the remaining 5 samples were taken on Day 0 (see Table 2).
| ANALYSIS NOTE: |
| In order to establish equivalency of saliva cortisol data across PCS2, PMBC, and PCS3, the Aggregated Data Set includes data for only 7 of the 11 PCS2 pre-challenge saliva samples and for 8 of the 14 PCS2 quarantine day 0 samples. Moreover, corresponding pre-challenge and day 0 area-under-the-curve (AUC) values appearing in the Aggregated data set were computed using 7 and 8 samples, respectively. Thus, AUC values for PCS2 participants are not completely comparable across the stand-alone PCS2 and Aggregated data sets. Additional information can be found in the section on Combining the 5 Studies. |
Assay Procedures: PMBC
Cortisol levels in saliva were determined using a competitive enzyme-linked immunosorbant assay (ELISA) procedure. Aliquots of each saliva sample were added to individual wells of a 96-well plate that had been pre-coated with rabbit antibodies to cortisol (rabbit anti-cortisol). Samples were then incubated to allow any cortisol present in the sample to bind to the rabbit antibodies. Following incubation, a solution containing a known amount of cortisol that had been labeled with horseradish peroxidase (HRP) was added to each well. The samples incubated for a second time, which allowed the HRP-labeled cortisol to bind to any rabbit antibodies that were not bound to sample-derived cortisol. The wells were then washed extensively to remove any unbound cortisol or HRP-labeled cortisol and a substrate solution was added to each well. If present due to binding of the HRP-cortisol to the bound antibody, the HRP would cleave the substrate, resulting in a color reaction. The optical densities of the wells were measured to determine the amount of substrate that was cleaved. There is a direct correlation between the amount of bound HRP-cortisol and the optical density of the well solution. Thus, the amount of cortisol present in the sample was inversely proportional to the amount of bound HRP-cortisol.
Reliability estimates for the salivary cortisol ELISA were established by examining curve-fitting for standard values and retesting previously run or known samples. These checks were conducted periodically, approximately every other month. Standard curves were good, and correlations between re-run samples and original values all exceeded .96. Average deviations between individual pairs of replicates were 4%.
PMBC cortisol assays were performed by the Immunologic Monitoring and Cellular Products Laboratory (IMCPL) at the University of Pittsburgh Cancer Institute.
Assay Procedures: PCS2 & PCS3
Cortisol levels in saliva were determined by time-resolved fluorescence immunoassay with a cortisol-biotin conjugate as a tracer1. Intra- and inter-assay variabilities were each less than 12%. PCS2 and PCS3 cortisol assays were performed by the laboratory of Dr. Clemens Kirschbaum, Dresden, Germany.
References
1. Dressendorfer, R. A., Kirschbaum, C., Rohde, W., Stahl, F., & Strasburger, C. J. (1992). Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. Journal of Steroid Biochemistry and Molecular Biology, 43, 683-692.
2. Walker, R. F., Joyce, B. G., Dyas, J., et al. (1984). Salivary cortisol: 1. Monitoring changes in normal adrenal activity. In G. F. Read, D. Riad-Fahmy, R. F. Walker, et al. (Eds.), Immunoassays of Steroids in Saliva (pp. 308-316). Cardiff: Alpha Omega.
3. Walker, R. F., Riad-Fahmy, D., & Read, G. F. (1978). Adrenal status assessed by direct radioimmunoassay of cortisol in whole saliva or parotid saliva. Clinical Chemistry, 24, 1460-1463.