Hair Cortisol (PCS3, subsample)
Collection of hair samples for the measurement of cortisol began at the start of Trial 8 and continued through the end of the study (Trial 13). Because providing hair samples was not a required component of the overall study protocol, participants were given the option of volunteering (or not) for this specific study activity. Volunteers completed a separate consent document pertaining specifically to the provision of hair samples for cortisol measurement, and received compensation commensurate with the number of samples they provided (1 or 2). A total of 36 participants volunteered for this component of the study.
Sample Collection
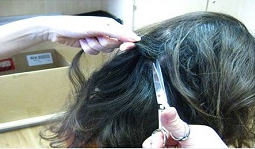
Hair samples for measurement of cortisol were obtained on two occasions: during the physical exam; and on the first day in the hotel, prior to viral challenge. Hair strands were collected from the back of the participant’s head, approximately level with the tops of the ears. If a participant lacked hair at this location, hair was collected from an adjoining area, with the actual location of sample collection being recorded. Strands were measured to ensure a sample length of at least 3 cm (1.2 in), and then cut with fine scissors as close to the scalp as possible. To avoid leaving a bald patch, several separate strands were obtained from an area of scalp no wider than 6 cm (2.4 in) in diameter. Individual strands were bundled immediately to avoid confusion regarding which end of the strands originated closest to the scalp. The final bundle was approximately the thickness of a standard pencil. Bundled hair strands were wrapped in tissue paper that had not been treated with any chemicals or dyes, secured with wire, labeled with an X at the scalp end, and then placed in a sealed plastic bag.
Hair cortisol extraction and measurement procedures were performed in the laboratory of Clemens Kirschbaum, PhD, Technical University of Dresden, Dresden, Germany.
Cortisol Extraction
The protocol of Davenport et al.1 was employed for washing of hair samples and extraction of cortisol. Briefly, each hair segment was placed in a 15 ml Falcon tube, 2.5 ml isopropanol was added, and then the tube was gently mixed on an overhead rotator for 3 minutes.
After having been allowed to dry for at least 12 hours, hair samples were powdered using a Retsch ball mill (5 minutes at 30 Hz). Fifty milligrams of powered hair was carefully weighed out and transferred into a 2 ml cryovial (Eppendorf, Hamburg, Germany), and 1.5 ml of pure methanol was added. Vials were then slowly rotated for 24 hours for cortisol extraction. After 24 hours, samples were spun in a microcentrifuge at 10.00 rpm for 2 minutes, and 1 ml of the clear supernatant was transferred into a new 2 ml cryovial. The alcohol was evaporated at 60°C under a constant stream of nitrogen for approximately 20 minutes. When the samples were completely dry, 0.4 ml of phosphate buffer was added and the tube vortexed for 15 seconds.
Assay
Cortisol concentrations were determined using a commercially available immunoassay with chemiluminescence detection (CLIA, IBL-Hamburg, Germany). The intra- and interassay coefficients of variation for this assay are < 8%.
References
1 Davenport, M. D., Tiefenbacher, S., Lutz, C. K., Novak, M. A., & Meyer, J.S. (2006). Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and Comparative Endocrinology, 147, 255-261.
2 Kirschbaum, C., Tietze, A., Skoluda, N., & Dettenborn, L. (2009). Hair as a retrospective calendar of cortisol production-increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology, 34, 32-37.