Standard Screening Measures
All 5 studies conducted a battery of standard screening measures to determine volunteers’ eligibility to participate in the research. These included blood chemistries, complete blood counts, pregnancy tests for female participants, urinalysis, and screening for human immunodeficiency virus (HIV) seropositivity 1. Additional study-specific screening measures (e.g., pre-challenge viral specific antibody titer and pre-challenge infection with wild viruses) were conducted in some of the studies. Unless otherwise indicated, biological specimens to be used for screening purposes were obtained at the follow-up screening physical (approximately 6-8 weeks prior to quarantine) or, for BCS, on admission to the Common Cold Unit (CCU; i.e., Quarantine Days -2 and/or -1). Because these measures were taken primarily to determine eligibility, most of the collected data are not available in the Common Cold Project data set. The two exceptions to this rule include data collected as a part of participants’ blood chemistries and complete blood counts, respectively.
With the exception of HIV seropositivity, abnormal laboratory results were followed up with a second test to confirm the abnormal value. Whether an exclusion criterion was met was based on the outcome of the confirmatory assay. Persons screening positive for HIV antibody were excluded from participation immediately following a first positive test result. However, in addition to being provided with their results, those testing positive were given instructions on getting re-tested and on obtaining HIV counseling.
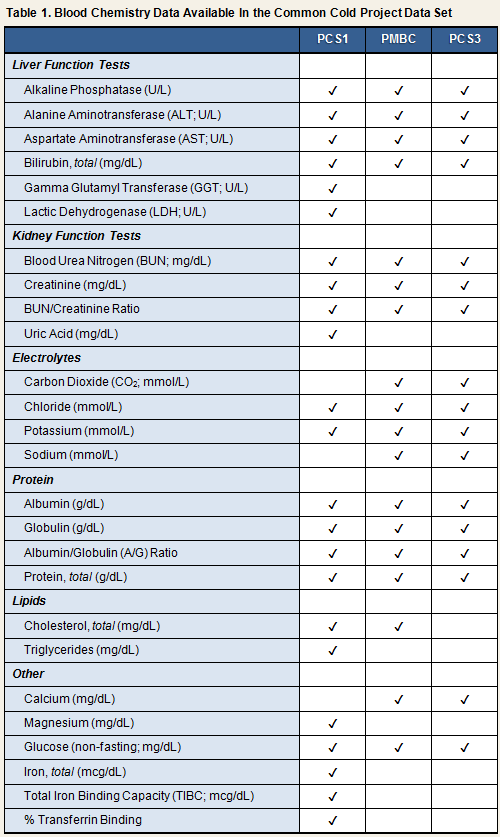
Blood Chemistry (BCS, PCS1, PCS2, PMBC, PCS3)All studies conducted routine blood chemistry screenings. Screenings conducted during PCS1, PMBC, and PCS3 included assessments of liver enzymes, renal function indices, electrolyte levels, total protein and albumin, and non-fasting glucose 2. Volunteers were excluded from participation in the study if their results indicated abnormal levels of any of the measured metabolic markers. In PCS2, PMBC, and PCS3, samples for assessment of blood chemistry were drawn at the screening physical exam; in PCS1, samples were drawn approximately 2 weeks prior to quarantine; and in BCS, on admission to the CCU. Blood chemistry analyses for PCS1 were performed by SmithKline Laboratories, and for PMBC and PCS3 by Quest Diagnostics. Laboratory information is not available for BCS and PCS2. Blood chemistry data from 3 of the 5 cold studies (PCS1, PMBC, and PCS3) are included in the Common Cold Project data set. Table 1 lists which blood chemistry measures are available for each study.
|
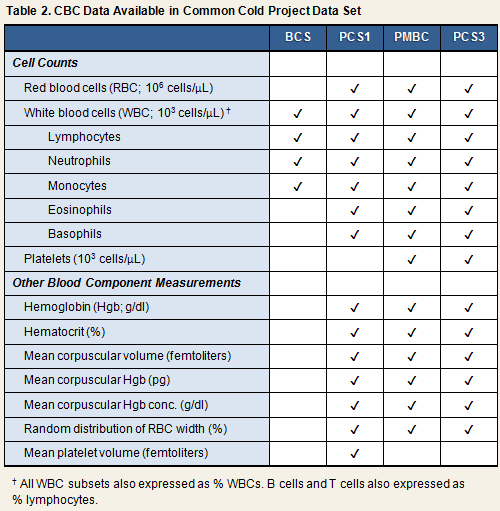
Complete Blood Count (CBC) (BCS, PCS1, PCS2, PMBC, PCS3)Screening CBCs were conducted in all 5 cold studies. For the 3 Pittsburgh cold studies and PMBC, these included counts of total white blood cells, white blood cell subsets, total red blood cells, hemoglobin concentration, hematocrit, and mean corpuscular volume and hemoglobin. The PMBC screen also included platelet count. For BCS, the screen included, at minimum, counts of total white blood cells and select white blood cell subsets 3. Volunteers were excluded on the basis of any abnormal result. Blood samples for CBCs were drawn simultaneously with those for blood chemistries. CBCs for PCS1 were performed by the Department of Hematology, University of Pittsburgh Medical Center and for PMBC and PCS3 by Quest Diagnostics. Laboratory information is not available for BCS and PCS2.
|
Pregnancy Tests (BCS, PCS1, PCS2, PMBC, PCS3)In the 4 Pittsburgh studies, female participants were asked to take a urine pregnancy test at the follow-up screening physical exam (6-8 weeks before viral challenge) and on Quarantine Day 0 (prior to challenge). For BCS, only a single test was administered on admission to the CCU due to the lack of a lengthy pre-quarantine baseline data collection period. In PCS3, pregnancy tests also were given immediately prior to the two stress reactivity sessions, which were conducted within 3 weeks pre-quarantine and approximately 4 weeks post-quarantine, respectively. Pregnancy tests were self-administered using an over-the-counter Human Chorionic Gonadotropin (hCG) detection kit, and results were read by study staff. Female volunteers who tested positive at screening were ineligible to enroll in the study; those who tested positive on Day 0 or at either of the 2 stress reactivity sessions (PCS3 only) were excluded from further participation. |
Urinalysis (BCS, PCS1, PCS2, PMBC, PCS3)In the 4 Pittsburgh studies, urine samples for urinalysis were collected during the screening physical exam and in BCS on admission to the CCU. BCS screened for the presence of glucose and protein in urine. PMBC and PCS3 employed a standard urine panel that included assay for the presence of bilirubin, ketones, glucose, protein, hemoglobin, nitrite, and leukocyte esterase 4. Volunteers were excluded from participation in the study if their results indicated abnormal presence of any of the measured analytes. Urinalyses for PMBC and PCS3 were conducted by Quest Diagnostics. Laboratory information is not available for the three earlier studies. |
Human Immunodeficiency Virus (HIV) Antibody (PCS1, PCS2, PMBC, PCS3)Prior to being screened for the HIV antibody, individuals who provided written informed consent to participate in the full study underwent an additional consent procedure (for which a separate consent form was required) after having their questions answered by the study physician. Serum for HIV antibody screening was obtained from blood samples drawn 2 weeks (PCS1), 4-6 weeks (PMBC), or 7-8 weeks (PCS2 and PCS3) before quarantine, using standard venipuncture techniques. HIV screenings were conducted by the Central Blood Bank for PCS1 and by Quest Diagnostics for PMBC and PCS3. Laboratory information is not available for PCS2. |
Serum Specific Antibody to the Challenge Virus (PMBC, PCS3)In addition to the baseline and post-challenge samples, PMBC and PCS3 included a screening sample that was collected 2 months prior to viral challenge. Based on this sample, volunteers were excluded from participation if their serum specific Ab titers were >4 (see Baseline & Post-Challenge Specific Ab to Challenge Virus, serum). None of the other three cold studies employed exclusion criteria based on specific Ab levels measured prior to viral challenge. Pre-challenge Ab screening assays were conducted in the laboratory of Ronald B. Turner, MD, Department of Pediatrics, University of Virginia Health Sciences Center. |
Screening for Pre-Challenge Infection with Nonspecific 'Other' Respiratory Viruses (PCS1, PCS2, PMBC-rhinovirus trials, PCS3)In the 4 Pittsburgh studies, nasal secretions also were assayed for the presence of respiratory virus strains other than the challenge virus. If a non-challenge virus strain was recovered from a participant’s pre-challenge (Quarantine Day 0) or post-challenge Day 1 nasal secretions, then all viral challenge data from that participant were treated as missing 5. ProtocolAfter frozen nasal lavage samples were quickly thawed in a 36°C water bath, 200 μl of lavage fluid was added to 2 tubes each of diploid fibroblast (MRC-5), epithelial (A-549), and kidney (PRMK) cells. Sample tubes and an additional 2-4 control tubes per each cell line were then placed in a roller drum at 36°C. |
Notes
1 HIV testing began with PCS1. Data from the British study were collected before HIV testing became widely available.
2 Details on which metabolic markers were included in the blood chemistry screenings conducted in BCS and PCS2 are not available (see Frequently Asked Questions).
3 Complete information on all blood components included in the BCS complete blood count screen is not available (see Frequently Asked Questions).
4 Details on which analytes were included in the urinalyses conducted in PCS1 and PCS2 are not available (see Frequently Asked Questions).
5 Because up to 2 weeks may be necessary to culture rhinovirus from a biological specimen, whether participants were infected with a non-challenge rhinovirus strain (wild virus) on Day 0 or Day 1 was not known until after the period of quarantine had ended. Thus, viral challenge data were collected on all participants, regardless of their Day 0 or Day 1 status, and only later excluded if a wild virus had been isolated.