Mind-Body Center Study
Study Description
The Pittsburgh Mind-Body Center (PMBC) Study was a prospective viral challenge study conducted from 2000-2004 among healthy volunteers ages 21-55 (mean age 37.3; SD 8.8). This study included detailed daily interviews with participants over 14 consecutive days to assess social interactions (number of interactions, with whom they were interacting, etc.), mood, and health behaviors. PMBC also included in-depth measurement of various aspects of marital relationships, including relationship satisfaction, spousal social support, marital commitment, and spousal self-disclosure. In addition (as part of the broader Mind-Body Center Project) numerous other psychological and behavioral variables were assessed including personal attributes, social factors, socioeconomic status, and health practices. Biological assessments before viral challenge included epinephrine, norepinephrine, cortisol, and stimulated cytokine production. Post-challenge measures, in addition to standard virology, included local (nasal secretions) cytokines (interleukin [IL]-1β, IL-6, IL-8, IL-10, IFN-α, and TNF-α).
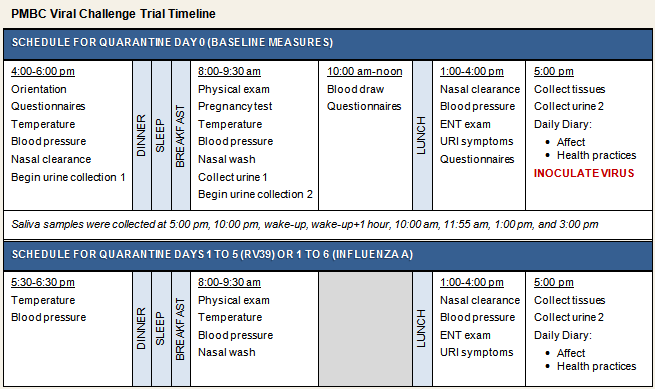
Participants were 95 men and 98 women who responded to advertisements and were judged to be in good health. Of these, the first 38 to be enrolled in the study were exposed to influenza A/Texas/36/91; all subsequent participants (n = 155) were exposed to rhinovirus (RV) 39. To maximize the rate of infection, only volunteers with viral-specific antibody titers ≤4 were deemed eligible for participation in the study. Prior to enrollment, volunteers completed a telephone screening, and screened participants were followed up with an in-person health evaluation by a study physician to further assess eligibility (see Human Subjects for information on additional inclusion and exclusion criteria). After completing baseline psychosocial and biological assessments, participants were administered nasal drops containing the challenge virus, followed in quarantine for either 5 (for RV39) or 6 (for influenza) days, and monitored for development of infection and objective signs of illness (see viral challenge timeline below). Approximately 28 days after virus exposure, blood was collected for serological testing. Participants were considered to have a cold if they both were infected with the challenge virus and met illness criteria. All individuals who completed the study received $800 for their participation.