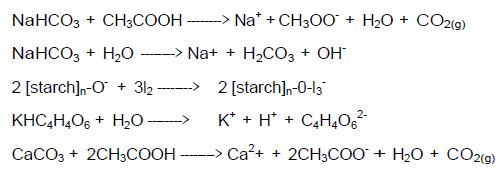Suspicious Groceries: Teacher's Notes
EQUIPMENT AND REAGENTS
- Jars for white powders
- Plastic sheet protectors for data sheets (or laminated data sheets)
- Dropper bottles for reagents
- Straw "Scoops"
- Litmus Paper
- Stirring rods (coffee stirrers)
- Safety glasses
- Iodine
- Vinegar
- Deionized water
- Corn starch
- Baking soda
- Powdered sugar
- Cream of tartar
- Calcium carbonate (chalk)
SOLUTION PREPARATION
Vinegar - Purchase white vinegar in the grocery store (5% acetic acid).
Iodine - Purchase iodine in a drug or grocery store and dilute 1 to 10 with deionized water.
This experiment makes use of standardized tests for acids, bases, and starches to determine the identities of unknown white powders. All of the powders are common household items, even the calcium carbonate, commonly known as chalk. White blackboard chalk can be finely ground and used for the experiment.
Expected results and the key to matching the drug name to the powder used are listed in the following table. (-) notes a negative test result. See also the photo in the lab introduction page.
Test Results
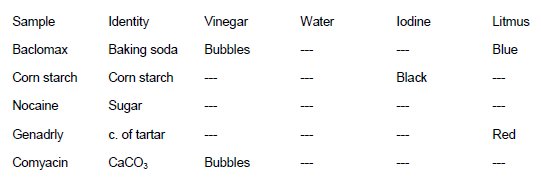
Any of the powders can be chosen as the unknown. Corn starch and baking soda have the most dramatic positive results. Only the calcium carbonate vinegar is subtle and must be carefully observed. Students should be told to look closely at the liquid on the plastic sheet. The tiny bubbles generated have a tendency to adhere to the plastic instead of foaming up like the baking soda-vinegar reaction. Unknowns can be put into numbered zip-lock bags for distribution to the individual students or groups.
Sample scoops are made out of plastic drinking straws. Cut each one into 3 pieces and then trim one end about 1/2 inch on a diagonal. Coffee stirrers can be used as stirring rods to mix the solutions if necessary. Often the liquids do not spread out very well on the powders.
Litmus paper should be of the type that indicates both acids and bases. This is usually called litmus neutral.
While these test procedures can be done by even the youngest students, they may not understand the chemistry that is taking place. This lab can be used to introduce them to the concepts of standardized tests and acids, bases, and starches. Upper level students can be required to explain the chemical reactions once the teacher reveals the actual composition of the powders. Equations for the positive test reactions are given at the end of this section.
Additional activities can include predicting the results of the same tests on other common "white powders", and testing food items for starch. Suggestions include pasta, potatoes, seeds, nuts, dairy products, and vegetables. Also include some non-food items like inexpensive paper plates and plain writing paper which are often stiffened with starch.
POSITIVE REACTIONS