Battery Workshop
Download lesson plan [zip]
Background
A battery is a portable device that stores chemical energy and is able to convert that stored chemical energy into electricity (Bates, Mary).
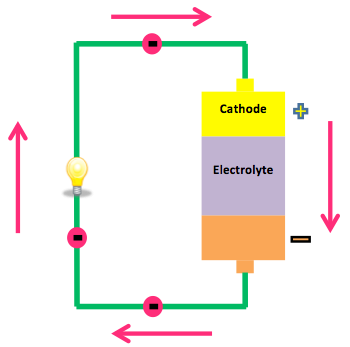
There are three main components to a battery: an anode, a cathode, and an electrolyte. The anode is the negative side of the battery and the cathode is the positive side of the battery. Both the anode and cathode are submerged in and separated by an electrolyte, such that the anode and cathode are not directly touching (UC Davis Chem Wiki). In the anode, a chemical reaction (oxidization) causes a buildup of electrons. These electrons want to travel to the electron-less cathode, but cannot travel through the electrolyte. Electricity is generated when a low-resistance circuit, such as a wire, connects the cathode and the anode. The electrons will travel along the circuit (current) to equilibrate the electron levels between the anode and cathode. As the electrons travel through the wire, they power the circuit, for instance providing electricity to light a light bulb. Eventually the battery will run out of power when the electrochemical reactions change the chemicals in the anode and cathode so that they no longer supply electrons.
Simple measurements can be performed on batteries to find the voltage and the current. Those measurements can be used to find the power generated by the battery. Specifically, the voltage (V) is equal to the product of the current (I) and resistance (R), or:
V=I*R
and the power (P) is equal to the product of the voltage and the current, or:
P=V*I=R*I^2
Objective
Students will be able to:
- Explain the structure of a battery and explain how it works.
- Explain the relationship between voltage, current, and power.
- Explain how much energy is actually stored in different types of batteries.
Materials Needed
- Computer
- Projector
- Lemons
- Zinc material (galvanized nail, zinc strip, zinc coin)
- Copper material (copper wire, copper strip, pre-1983 penny)
- Multimeter
- "Batteries Worksheet" [docx] [pdf]
Safety Concerns
Ensure all surfaces and hands are dry before working with the lemon batteries.
Vocabulary
- Battery: a portable device that stores chemical energy and is able to convert that stored chemical energy into electricity.
- Anode: the negative side of a battery where electron buildup occurs.
- Cathode: the positive side of a battery.
- Electrolyte: separates the anode and cathode
- Energy: the capacity of something to do work; an amount. Measured in watt-hours, kilowatt-hours, megawatt-hours. A typical American household used 940 kWh per month in 2011.
- Power: describes how much energy can be produced in a given time. Also to supply a device with electricity; the product of voltage and current. A common unit of measurement is a watt (W); also measured in watts, kilowatts, megawatts, etc.
- Voltage: the difference in the electric charge of two places. A common unit of measurement is a volts (V).
- Current: flow of electric charge, or the flow of electrons. A common unit of measurement is an ampere, or amp (A).
- Resistance: a material’s opposition to electric current. A common unit of measurement is an ohm (Ω).
Procedure
Activity 1: Introduction to Batteries
Time: 20 minutes
Supplies: Computer, projector
Description
- Introduce the topic of batteries. Suggested questions include:
- What are different ways that we get electricity?
- What are some things we use batteries for?
- Why do we use batteries? (Portability)
- Why don’t we use batteries to power everything? (Too expensive)
- Define and discuss what the cathode, anode, and electrolyte are.
- Discuss the process of generating electric power with a battery
- Show a diagram of a battery (see Figure 1 in background)
Activity 2: Lemon-based batteries
Time: 30 minutes
Supplies: Lemons, something copper, something zinc, multimeter,
Description
- Discuss how to use the multimeter .
- Show the students how to assemble a battery
- See how to here: http://www.wikihow.com/Create-a-Battery-from-a-Lemon and http://www.youtube.com/watch?v=GhbuhT1GDpI
- A copper penny (before 1982), copper wire, or a copper strip can be used for the copper side of the battery. A galvanized nail, a zinc strip, or a zinc coin can be used for the zinc side of the battery.
- Pair the students off and give them the materials to create the lemon based batteries
- Have students measure the voltage and current from the lemon-based batteries.
- Record the voltage / current measurements.
- Have the lemon batteries light up a few LEDs, to prove that the batteries actually work.
Activity 3: Final discussion
Time: 30 minutes
Supplies: "Batteries Worksheet" [docx] [pdf]
Description
- Have the students work in their pairs (and with other groups, if needed) to complete the batteries worksheet. Students are not expected to be able to answer everything.
- Discuss the lemon batteries, and how they differ from the batteries discussed before.
- Discuss battery structure again and how it relates to the lemon battery
- Calculate the power from the lemon batteries using the voltage and current measurements
- Discuss how power outputs of different batteries will vary, and how the power from the lemon battery compares to power requirements. Suggested questions include:
- How many lemon batteries would be needed to power a light bulb? A cell phone? A laptop? A car? (from worksheet)
- What is the difference between lemon batteries and manufactured batteries? (Not much, just better design)
Additional Resources
Reputable
DOE – Batteries. "Batteries." Department of Energy. Department of Energy. Web. 20 Jun 2013. <http://energy.gov/articles/building-better-battery-vehicles-and-grid>.
The DOE’s page on batteries offers a series of articles introducing their work with batteries (specifically in vehicles), as well as articles about their work on related topics. Any teachers looking for current work done with batteries and vehicles could give this page a browse.
UC Davis Chem Wiki. "Batteries: Electricity through chemical reactions." UC Davis Chem Wiki. UC Davis.
Web. 28 Jun 2013. <http://chemwiki.ucdavis.edu/Analytical_Chemistry/Electrochemistry/Voltaic_Cells/Batteries:_Electricity_though_chemical_reactions>
The UC Davis page – adopted from two textbooks – gives an in depth look at batteries and related topics, such as battery history, types of batteries, and hazards associated with batteries. The set up is very similar to a Wikipedia page. Teachers looking for a wide array of battery information from reputable sources – or teachers looking for textbooks to provide more information – could look here.
Electric Power. "Electric Power." GSU. Georgia State University. Web. 28 Jun 2013. <http://hyperphysics.phy-astr.gsu.edu/hbase/electric/elepow.html>.
This site talks about the relationship between power, voltage, and current, as well as how the concept of power is applied to resistors and circuits. Teachers looking to expand on the topic of power and do some
CMU Electric Vehicles Group. "Electrified Vehicles." Vehicle Electrification Group. Carnegie Mellon University. Web. 20 Jun 2013. <http://www.cmu.edu/cit/veg/electrified vehicles/index.html>.
CMU’s page on electric vehicles gives a brief description of hybrid electric vehicles, plug-in hybrid electric vehicles, and battery electric vehicles. Teachers looking to learn more about batteries within vehicles could look here for examples of vehicle types.
Northwestern – How Batteries Work. "How do batteries work?." Power System. Northwestern University. Web. 19 Jun 2013. <http://www.qrg.northwestern.edu/projects/vss/docs/power/2-how-do-batteries-work.html>.
Northwestern’s succinct guide to how a battery works is made for a younger audience. It includes a brief description of how it works, a diagram, and links to related topics explained in a similarly simple way. Teachers looking for a good outline as to what can be explained easily, or a way to explain batteries in a more elementary way
MIT Ask An Engineer – How does a battery work? Bates, Mary. "How does a battery work?." MIT Engineering - Ask An Engineer. MIT, 1 May 2012. Web. 19 Jun 2013. <http://engineering.mit.edu/live/news/2037-how-does-a-battery-work>.
The MIT engineer explains how a battery works with a little more technical terminology than other resources on this page, but still gives a relatively simple explanation of both disposable and rechargeable batteries. Teachers looking for a more sophisticated way to phrase something may want to look here.
Impact Assessment of Selected Policy Options for Revision of the Battery Directive. "Impact Assessment on Selected Policy Options for Revision of the Battery Directive." BIO Intelligence Service. European Commission, n.d. Web. 20 Jun 2013. <http://ec.europa.eu/environment/waste/batteries/pdf/eia_batteries_final.pdf>.
The European Commission Final Report on how different policy options affect batteries and accumulators. While very dense and abundant with data, teachers looking to connect battery technology and policy could look here.
National Renewable Energy Lab – Batteries. "NREL: Energy Storage - Batteries." National Renewable Energy Laboratory. National Renewable Energy Laboratory, 30 Jan 2013. Web. 20 Jun 2013. <http://www.nrel.gov/vehiclesandfuels/energystorage/batteries.html>.
The batteries of hybrid electric, electric, and fuel cell vehicles are all discussed in great technical detail on the NREL battery page. There is also a quick description of how a lead battery works, accompanied with an illustration. Teachers looking for a technical application of batteries may want to look here.
DOE – Vehicle Technologies Office. "Vehicle Technologies Office." Department of Energy. Department of Energy, 15 Apr
2013. Web. 20 Jun 2013. <http://www1.eere.energy.gov/vehiclesandfuels/technologies/energy_storage/>.
A brief description on the different type of vehicle technologies is given on this page. The DOE also describes more of the work they work trying to improve vehicle battery performance. Teachers looking for information about actual work being done with the technology could work here.
Opinion / Newspaper
ABB. "ABB Converters for Battery Energy Storage." ABB. ABB. Web. 20 Jun 2013. <http://www.abb.us/product/us/9AAC167809.asp>
ABB gives a brief description of the electrochemical nature of batteries, and then talks a bit about the role of the battery energy storage system they’re working on. If a teacher wants to demonstrate a more unique usage of batteries in power generation, they may want to look here as an example.
AllAboutBatteries.com. "Battery energy storage in various battery types."AllAboutBatteries.com. AllAboutBatteries.com. Web. 20 Jun 2013. <http://www.allaboutbatteries.com/Battery-Energy.html>.
The AllAboutBatteries page talks about the stored energy in batteries in terms of units (joules) and offers other links on a sidebar about battery history and other related topics. If a teacher wanted to focus more on the energy content of batteries, instead of how the energy is generated, this may be a good place to look.
Energizer Interactive Diagram. "How do Alkaline Batteries Work?." Energizer. Energizer. Web. 19 Jun 2013. <http://www.energizer.com/learning-center/Pages/how-batteries-work.asp>
Energizer provides an interactive, diagram-filled explanation on how batteries work. This includes the history of the battery, how to construct a battery, and how a battery works in an application (in this case, the flashlight). Teachers looking for good visual aids or for a good example of how a battery works should look here.
How Stuff Works – How Batteries Work. Marshall Brain, Charles W. Bryant, and Clint Pumphrey. ""How Stuff Works" How
Batteries Work." How Stuff Works. How Stuff Works, n.d. Web. 19 Jun 2013. <http://electronics.howstuffworks.com/everyday-tech/battery.htm>.
The How Stuff Works page on batteries is a comprehensive guide to battery history, how batteries work, suggested experiments, and additional sources. Teachers looking for a good, easy to read, page to begin their research could begin here.
Energy Quest Chapter 5. "The Energy Story - Chapter 5: Stored Energy and Batteries." Energy Quest. Energy Quest, n.d. Web. 20 Jun 2013. <http://www.energyquest.ca.gov/story/chapter05.html>.
The Energy Quest page on batteries gives a unique explanation about the chemistry behind how a battery works while still keeping a simple explanation. Teachers looking to focus more on the chemistry instead of talking strictly about the electron flow could look here.
Wikihow – Lemon Batteries. "5 Tips on How to Create a Battery from a Lemon."WikiHow. WikiHow. Web. 5 Jul 2013. <http://www.wikihow.com/Create-a-Battery-from-a-Lemon>.
This WikiHow page on batteries gives very clear, simple instructions on how to create a lemon battery. This is as good a resource as any to see the steps involved in making a lemon battery on your own.
Batteries – Wikipedia. "Battery (electricity)." Wikipedia. Wikipedia - The Free Encyclopedia, 30 May 2013. Web. 20 Jun 2013. <http://en.wikipedia.org/wiki/Battery_(electricity)>
The Wikipedia page on batteries gives an introduction on how batteries work as well as information on their history, different types of batteries and battery waste. For teachers looking to get a general oversight about batteries, as well as access to other sources, Wikipedia might be a good starting place.
Other
Video - How a lemon battery works
Youtube Video – How Batteries Work. “How do batteries work – Science Videos for Kids.” Online video clip. Mocomi Kids.
Youtube. 14 February 2012. Web. 19 June 2013. http://www.youtube.com/watch?v=KkRwuM4S8BQ
This video gives a colorful, well-diagramed explanation as to how batteries work. The video is clearly kid-friendly, so any teachers looking to give a quick introduction to younger students could look here.
Author(s)
Lesson idea from Eric Hittinger; final product compiled by Sabrina Larkin on behalf of the Leonard Gelfand Center for Service Learning and Outreach.
Funding Sources
Portions of this work were supported by a) the Leonard Gelfand Center for Service Learning and Outreach, and b) the Center for Climate and Energy Decision Making (SES-0949710) through a cooperative agreement between the National Science Foundation and Carnegie Mellon University.
Next Generation Science Standards Alignment
HS-PS3-3: Design, build, and refine a device that works within given constraints to convert one form of energy into another form of energy.
Crosscutting Concepts: Systems and System Models (Predicting the behavior of a system)
Connections: HS.ESS3.A
Last updated: September 19, 2013