Polymer Structure Activites
The mobility of molecules that consist of long chains of atoms connected together is very different from that of a small molecule. This can be demonstrated using different activities depending on the level of the student.
The first activity is best suited for elementary school children. Have the children stand and pretend to be a small molecule, by moving about freely near their desk. To form a long chain polymer, the students then connect their hands to form a line or "chain" (you should have at least 2 chains). Now have them move about without letting go of their neighbor's hand--have them comment about how less "free" they feel, compared to when they were a small molecule.
For middle school children, 'polymer' chains can be formed by interlocking paper clips together (each paper clip is a "monomer") and noting the difference in how a chain of paper clips can be moved about compared to a single paper clip. This is a two-dimensional model of a polymer. If paper clips are not available, you can use strips of paper to form circles, connecting one circle to the next to make a paper chain.

Suggest that students compare how free the single paper clip (or paper circle) is to move compared to a paper clip or paper circle that is part of a chain structure.
For high school students, the polymer chemistry can be presented in more detail. An example using ethylene monomer, C2H4, can be used to illustrate this concept. A polymer results when the monomers are reacted (connected) together. In the case of ethylene, the result is polyethylene.
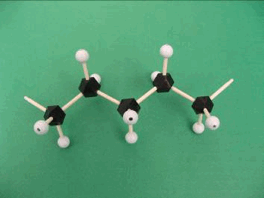
A simple three dimensional model can also be constructed using gum drops and toothpicks, or a molecular model set. The sequence of photos below shows how this can be done.

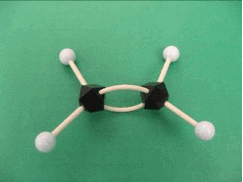
The small light blue balls and black polygons can be taken to represent two different types of atoms (for example, hydrogen and carbon, respectively). The sticks will be used to represent connections or bonds between the atoms. A small molecule like ethylene can be built easily using these objects. The bent connectors represent a double bond between the carbon atoms.
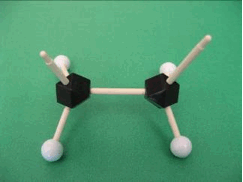
In order to connect 2 ethylene molecules together, we need to convert the molecule into a monomer that is ready to react. Below you can see the ethylene monomer with 2 unconnected/free bonds.
Several monomers can now be connected to start to form the polymer chain. The photo below shows the beginning of a polyethylene chain. Depending on how many 'atoms' and 'bonds' are available, the students can create a fairly large polymer.