Polymer Architecture
A polymer was defined in the previous section as a macromolecule made up of many segments connected together. It is very common for these segments to contain what is called a carbon backbone. That is, Carbon atoms form the 'spine' of the polymer chain and other elements/molecules dangle from the Carbon atoms. The monomers pictured below illustrate this concept.
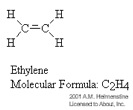
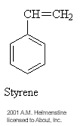
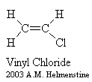
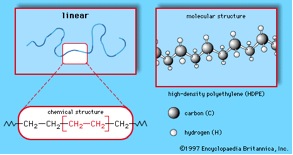
The process for connecting the segments together to form a polymer is called polymerization. Depending on the structure of the monomer and on the polymerization method employed, polymer chains may show different architectures. If the segments are connected through the Carbon atoms, then a linear polymer chain results. The high density polyethylene shown in the figure below is a good example of a linear polymer chain.
Sometimes a polymer chain can have segments branching off of the main carbon backbone. This structure is called a branched polymer chain. The following figure shows the case of low density polyethylene. Notice the ethylene segments dangling from the polyethylene chain.
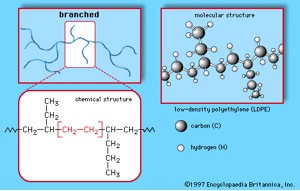
Branched polymer molecules cannot pack together as closely as linear molecules can; so the forces holding these polymers together tend to be much weaker. This is the reason why the highly branched low density polyethylene is very flexible and is used as packaging film (e.g. Saran Wrap®) and plastic grocery bags; while the linear high density polyethylene is tough enough to be shaped into objects such as bottles or toys.
When the branches on a polymer chain further react/connect with neighboring chains, the result is a network structure (ladder-like). The figure below shows a phenol-formaldehyde polymer. This material is formed when molecules of phenol (C6H5OH) are linked by formaldehyde (CH2O) to form a complex network of interconnected branches.
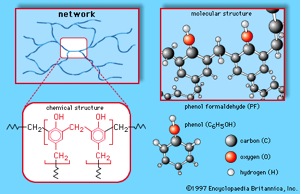
The connections between polymer chains that form the network structures can vary in strength. Strong connections result from actual chemical bonds (covalent and/or ionic), while weaker connections result from Hydrogen bonds and Van Der Waals interactions. Polymer networks containing chemical bonds are called cross-linked polymers. One example of a cross-linked polymer is vulcanized synthetic rubber, used in automobile tires. An example of a network polymer formed by weak connections is the gel formed by pectin. This natural network polymer is the basis for jellies and jams.
The properties of network polymers depend on the density of the network. Polymers having a dense network, like the example shown in the figure above, are very rigid and sometimes even brittle. Network polymers containing long, flexible branches connected at only a few sites along the chains are rubbery and exhibit elastic properties.