"Reactive" Block Copolymers
Surfactants
Surfactant for dispersion polymerization
Specifically designed for destruction of DNAPL
Reactive Surfactants
Block Copolymers that Provide Antibacterial Properties to Surfaces
Surfactants:
Three examples will be provided to illustrate how the components of each segment in a block copolymer can be selected to give the final material a set of properties required to accomplish a specific task. Indeed one of the earliest examples of a surfactant for a specific application was a report on molecular engineering a surfactant for a very specific task.(1) The preprint reported several synthetic routes to produce surfactants with properties tailored for use in supercritical CO2 based polymerization media including dispersion polymerization, cleaning processes and drug dispersions. Small angle neutron scattering (SANS) measurements show that these molecularly-engineered surfactants self assembled to form stable micelles in CO2. Surfactants were also developed to allow conducting an ATRP in supercritical CO2.(2)
Block copolymer specifically designed as a surfactant for dispersion polymerization:
Since the preparation of polymers with terminal functionality and block copolymers or segmented copolymers is "easy" by CRP techniques it would be expected that materials suitable as surfactants have been prepared and used in various applications including emulsion polymerization(3) and dispersion polymerization in super-critical CO2,(2) each of which required the synthesis of a specific fluorinated polymeric surfactant stabilizer.(1)This has indeed been the case, however, we will discuss a more recent example of a surfactant specifically designed for a particular application. This is the synthesis and use of a poly(ε-caprolactone)-b-poly(octadecyl methacrylate)-b-poly(dimethylaminoethyl methacrylate) block/random copolymer which was expressly designed and prepared to act as a surfactant in the dispersion polymerization of L,L-lactide(4,5) to form biodegradable nanoparticles of controlled dimensions. (6)
The poly(ε-caprolactone) block was prepared first by an anionic ring opening polymerization of ε-caprolactone initiated with hydroxyethyl 2-bromoisobutyrate/ tinII hexanoate system followed by sequential ATRP of octadecyl methacrylate then dimethylaminoethyl methacrylate.
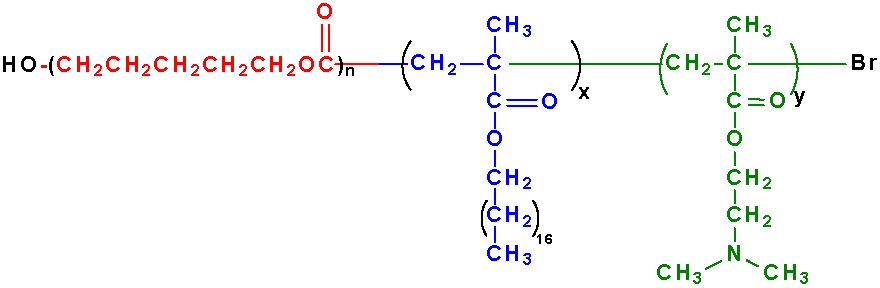
The ABC triblock copolymer functioned as a surfactant for the dispersion polymerization of cyclic esters and microspheres (particles with diameters above 0.5 mm) with pre-selected functional groups in the surface layer. The block copolymers could be prepared either directly in the presence of surfactants containing polyester blocks and blocks with the required terminal chemical groups or by post-treatment (partial hydrolysis) of the earlier synthesized polyester microspheres without functional groups.
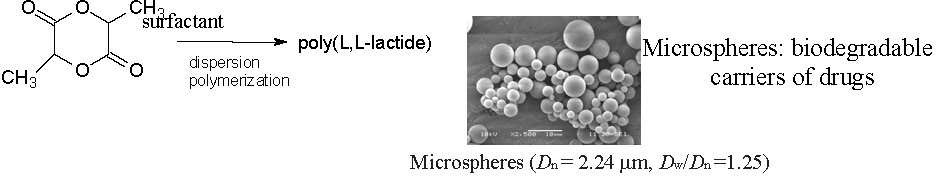
Hydrolysis introduced carboxyl and hydroxyl groups into surface layer of these particles. The microspheres were transferred (via ethanol) to water and did not aggregate due to stabilization by the DMAEMA blocks containing amino groups.
Block copolymer specifically designed for destruction of DNAPL:
DNAPL, or Dense Nonaqueous Phase Liquids, are mainly chlorinated solvents, e,g trichloroethylene, tetrachloroethylene or chloroform that have accumulated in aquifers . Iron nanoparticles are a good material to use when targeting destruction of DNAPL. The iron nanoparticles are environmentally friendly, commercially available at reasonable cost, ~$25/kg and have a large surface area for efficient interaction with the contaminants. Their downside is that they undergo rapid flocculation in aqueous systems and have little affinity for a DNAPL/water interface. To overcome these deficiencies a series of block copolymers were designed to deliver iron nanoparticles to DNALP in underground reservoirs where they can destroy the DNAPL via reductive dechlorination reactions.
The block copolymers have an anchoring segment, and both a hydrophobic and a hydrophilic block. The first amphiphilic ABC triblock copolymer precursors consisting of poly((tBMA)-b-(MMA)-b-(St)) and poly((tBMA)-b-(BMA)-b-(St)) were synthesized via ATRP. Sulfonation of the polystyrene block and hydrolysis of t-butyl ester groups were conducted simultaneously by reaction with acetyl sulfate.(7)
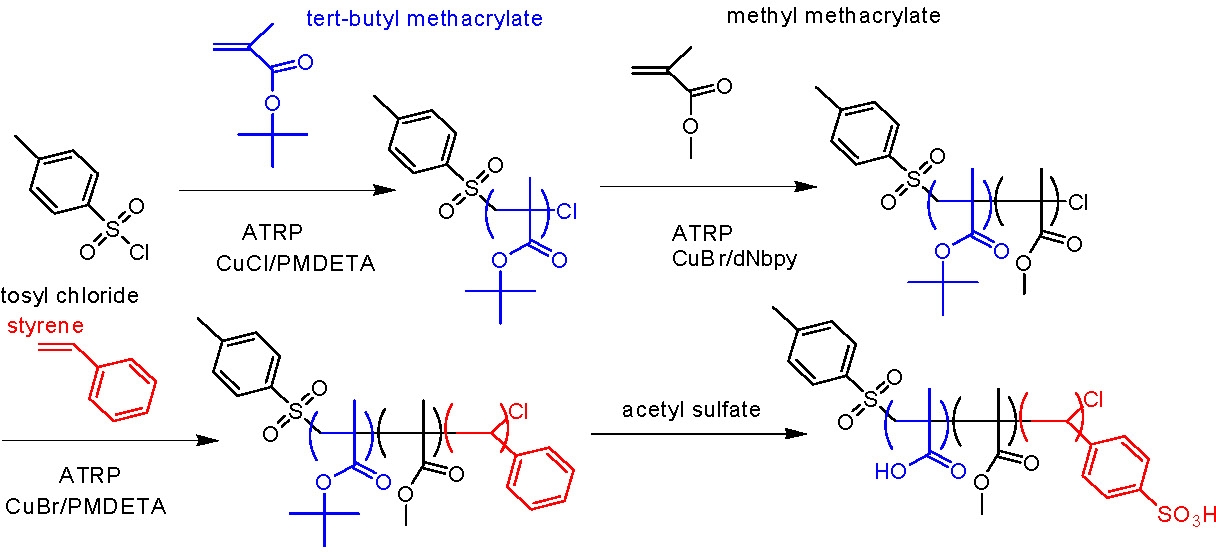
The resulting block copolymers were capable of self-attachment to iron nanoparticles by mixing commercially available iron nanoparticles with the triblock copolymer followed by sonication in water. A slight shift in size distribution of the iron particles was clearly observed but the size distribution remained monomodal making the composite structures suitable for transportation through aqueous media to hazardous DNAPLs in aquifers. When contact is made with a hydrophobic medium the hydrophobic block swells and releases the iron nanoparticle which could then enter and destroy the DNAPL via reductive dechlorination reactions.
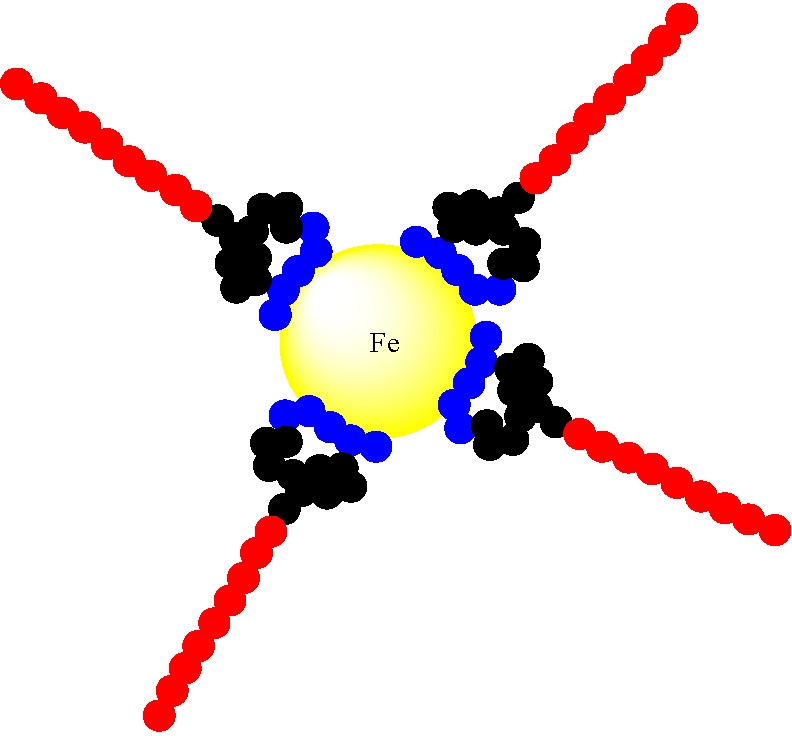
The delivery strategy is provided in the following schematic.
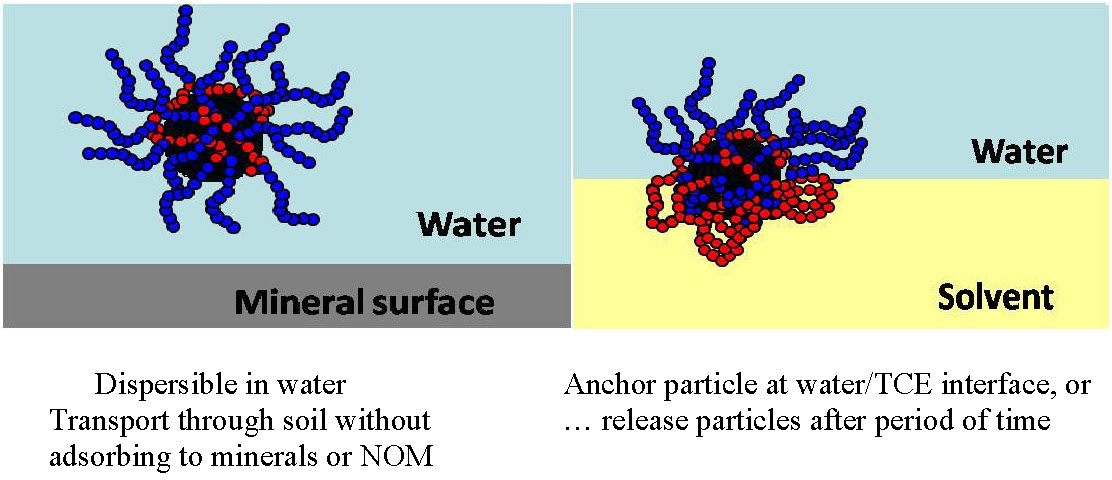
In bench top experiments the modified iron particles migrate to the interface between water and organic solvents.(8) This behavior is strongly dependent in the composition of the copolymer but the narrow size distribution of emulsion droplets is typical of stable Pickering emulsions and the emulsions are stable more than 6 months. Some of the parameters examined include the effect of polymer architecture and composition on: colloidal stability, the specificity of targeting DNAPL and microbial degradation of the surfactant.
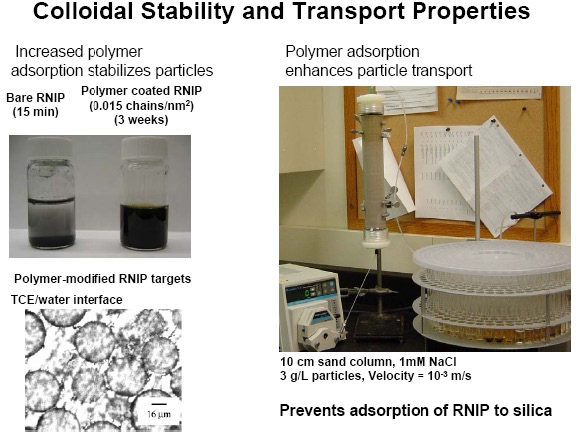
Therefore a targeted reactive nano-iron delivery system for the remediation of chlorinated solvent-contaminated groundwater was obtained and is being examined in real soil. The effect of particle concentration and solution ionic strength on the transport of each modified nanoiron particle was evaluated. The filtration mechanisms for bare and modified particles were determined in microfluidic flow cells and quartz crystal microbalance (QCM) experiments that probe the particle-collector grain interaction. The effect of surface modification on nanoiron reactivity was evaluated in batch experiments.
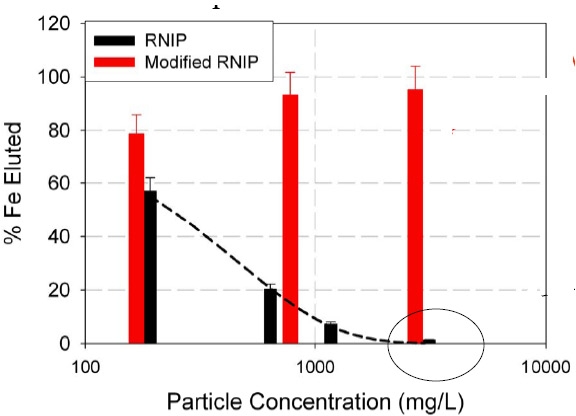
Polymer adsorption on the iron nanoparticles improved transportation of the particles through a bed of sand. Transport of modified nanoiron does not directly correlate with z-potential or colloidal stability, but rather correlates to particle-grain interactions. Filtration of bare nanoiron is caused by straining and subsequent clogging rather than by deposition to clean sand grains, suggesting that filter ripening models rather than clean bed filtration models should be used to describe nanoiron transport at high particle concentration. Surface modification decreased nanoiron reactivity by 2-4 times, but as high as a factor of 9 depending on the modifier used. Amphiphilic triblock copolymer modified nanoiron with a high hydrophobe/hydrophile ratio shows promise for in situ targeting of NAPL, but requires further optimization.(8)
Reactive Surfactants:
One of the limitations of conducting a CRP in miniemulsion or microemulsion systems is the requirement for high levels of surfactants that could contaminate the final high value nano-particles. This has been overcome by the preparation of reactive surfactants that act as initiators for the subsequent CRP.(9,10) In the later reference amphiphilic block copolymers poly(ethylene oxide)-b-polystyrene (PEO-PS-Br) with various molecular weight were synthesized using a poly(ethylene oxide) homopolymer (PEO-Br) macroinitiator and the used as AB macroinitiators and stabilizers (reactive "surfactants") for activator generated by electron transfer atom transfer radical polymerization (AGET ATRP) of Bu acrylate (BA), in miniemulsion either with or without Et 2-bromoisobutyrate (EBiB) as co-initiator. Under both conditions, the reactions were well controlled and stable latexes were formed. In the absence of EBiB, polymer particles with diameter around 120-230 nm were obtained, and the particles contained polymers with mol. wt. Mn = 16,000-25,000 g/mol and relatively low polydispersity (Mw/Mn = 1.2-1.4). The amount of reactive surfactant used can be reduced in the reaction (1.7-4 wt% vs monomer) in the presence of a low molecular weight initiator, EBiB. The percent of initiating sites from EBiB was changed from 30% to 90% when the majority of the polymers were initiated by EBiB. Nevertheless, because of the covalent linking of the surfactants to polymer chains, no free surfactant was left in the reaction system. The final diameter of latexes stabilized by reactive surfactants was around 200-390 nm, the number average of the molecular weight (Mn) of the polymers obtained was 10,000-25,000 g/mol, and the polydispersity index was around 1.2.
A similar approach has been employed for RAFT and NMP CRP processes. In the RAFT example an ab initio batch emulsion polymerization of styrene and butyl acrylate were conducted using an amphiphilic PEO macro-RAFT agent with a trithiocarbonate reactive group as both a stabilizer and a reversible chain transfer agent.(11) The surfactant free approach allowed easy formation of auto-stabilized latexes composed of diblock copolymers, with solids content as high as 24 wt %. The molar mass of the hydrophobic block and the size of the particles can be tuned by changing the macro-RAFT/monomer ratio. A Nitroxide-Mediated Controlled/Living Free-Radical Surfactant-Free Emulsion Polymerization of Methyl Methacrylate(12) the polymerization was conducted using a poly(methacrylic acid)-based macroalkoxyamine initiator.
Block Copolymers that Provide Antibacteial Properties to Surfaces:
Several block copolymers have been prepared displaying bactericidal properties both at CMU in conjunction with University of Pittsburgh (grafting from surfaces is described below) and other workers around the world. An example is a block copolymer designed for dispersion in a bulk polymer to modify surface properties(13) poly[2-(tert-butylamino)ethyl methacrylate] (PTBAEMA) belongs to a novel class of water-insoluble biocides. Dispersion of a poly(ethylene-co-butylene)-b-poly[2-(tert-butylamino)ethyl methacrylate] diblock copolymer (PEB-b-PTBAEMA) within low-density polyethylene (LDPE) imparts antimicrobial properties to the polyolefin as assessed by the viable cell counting method against Escherichia coli (E. coli). This diblock copolymer was synthesized by ATRP with a poly(ethylene-co-butylene) (PEB) oligomer end-capped by an activated bromide as a macroinitiator for the polymerization of 2-(tert-butylamino)ethyl methacrylate (TBAEMA). Morpholology changes of E. Coli bacteria in contact with modified LDPE have been observed by transmission and SEM and indicate that the diblock copolymer is a bactericide rather than a bacteriostatic molecule. The action mode of the PEB-b-PTBAEMA copolymer more likely relies on the displacement of the Ca2+ and/or Mg2+ ions in the outer membrane of the bacteria resulting in disorganization and finally disruption.
The reverse approach was also examined when reactive grafting onto a polyolefin was targeted.(14) In this example, which also sought to combine low cost, good mechanical properties, and antibacterial activity in one material, a nonquaternized polymeric biocide, i.e., poly[2-(tert-butylamino)ethyl methacrylate] (PTBAEMA), was dispersed within a commodity plastic, in this case, polypropylene (PP). The high immiscibility of the two polymers was tackled by reactive compatibilization. This was accomplished by reaction of commercially available maleic anhydride grafted polypropylene with primary amine-end-capped PTBAEMA. This telefunctional reactive polymethacrylate was synthesized by ATRP using an azide-containing initiator. The azide end group was converted into a primary amine by the Huisgen [3 + 2] cycloaddition of propargylamine. Accordingly the formed PP-g-PTBAEMA copolymer was melt dispersed within neat PP and processed as fibers, whose antimicrobial properties were assessed by the viable cell counting method against E coli. The antibacterial activity was long-lasting as a result of the anchoring of the PTBAEMA chains onto PP, which prevented them from being released from the surface of the fibers.
Grafting onto glass a surface is shown below.
In this instance the tethering segment was a trimentyoxysilylpoly(methacrylate).
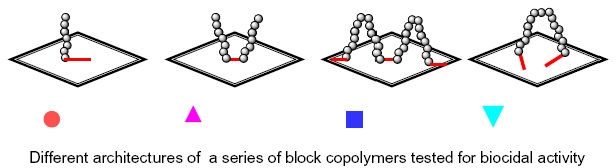
Several different polymer topologies were examined but the only critical parameter for biocidal action was the concentration of quaternizable poly(DMAEMA) that could be attached to the surface.
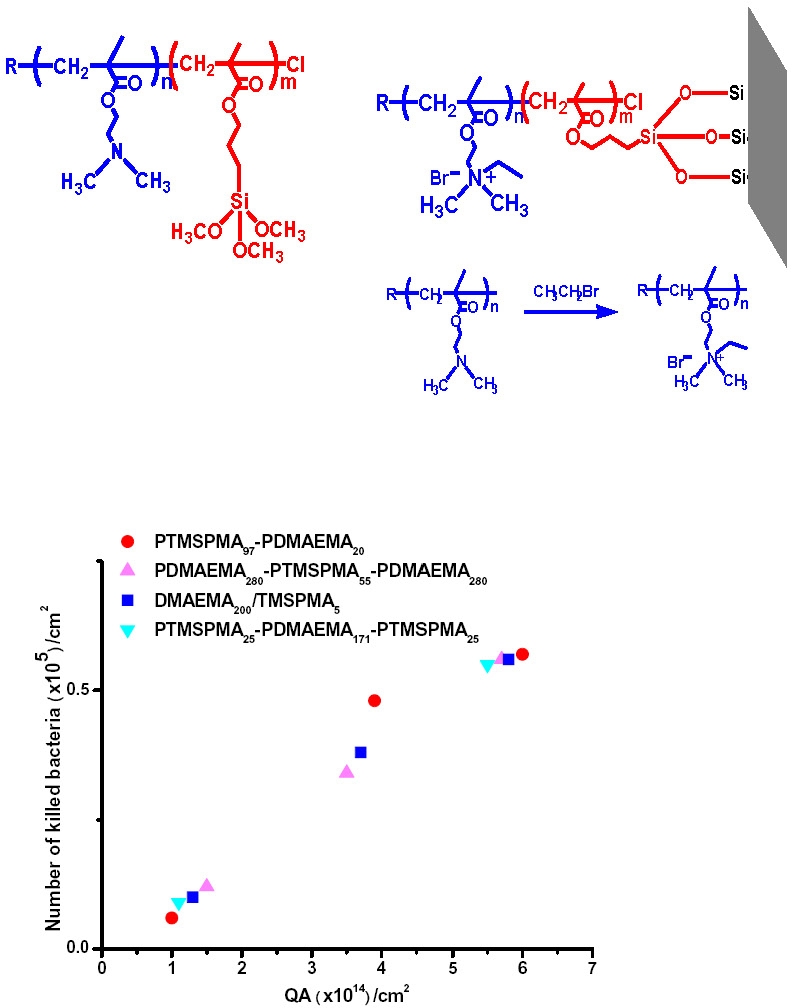
Since the length of the QA chains attached to the surface was lower than the MW required to puncture a cell wall the biocidal activity was due to an ion exchange mechanism.
Recent progress in the synthesis of so-called ‘schizophrenic' water-soluble block copolymers was reviewed by Armes.(15) The original report in this new sub-field of block copolymers involved a tertiary amine methacrylate-based A-B diblock copolymer synthesized by group transfer polymerization that was both pH- and salt-responsive, allowing the formation of either A-core or B-core micelles in aqueous solution. A second example involved a poly(propylene oxide)-tertiary amine methacrylate diblock copolymer synthesized via ATRP that exhibited both pH- and thermo-responsive behavior. More recently, several examples of wholly pH-responsive zwitterionic diblock copolymers (prepared via ATRP, usually using protecting group chemistry) have been reported, with their aqueous solution behavior being characterized with varying degrees of precision. The synthesis and characterization of purely thermo-responsive diblock copolymers is also discussed, along with the first example of an ABC triblock copolymer that is capable of forming a ‘trinity' of micelles in aqueous solution at 20 0C simply by adjusting the solution pH. In this remarkable final example, the cores of the three types of micelles are formed by hydrophobic forces, polyion complexation and hydrogen bonding, respectively.
REFERENCES
(1) DeSimone, J. M.; Betts, D.; Johnson, T.; McClain, J. M.; Wells, S. L.; Dobrynin, A.; Rubinstein, M.; Londono, D.; Wignall, G.; Triolo, R. Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.) 1999, 40, 435-436.
(2) Xia, J.; Johnson, T.; Gaynor, S. G.; Matyjaszewski, K.; DeSimone, J. Macromolecules 1999, 32, 4802-4805.
(3) Burguiere, C.; Pascual, S.; Coutin, B.; Polton, A.; Tardi, M.; Charleux, B.; Matyjaszewski, K.; Vairon, J.-P. Macromol. Symp. 2000, 150, 39-44.
(4) Jakubowski, W.; Lutz, J.-F.; Slomkowski, S.; Matyjaszewski, K. Journal of Polymer Science, Part A: Polymer Chemistry 2005, 43, 1498-1510.
(5) Jakubowski, W.; Matyjaszewski, K. Macromolecular Symposia 2006, 240, 213-223.
(6) Slomkowski, S.; Gadzinowski, M.; Sosnowski, S.; De Vita, C.; Pucci, A.; Ciardelli, F.; Jakubowski, W.; Matyjaszewski, K. Macromolecular Symposia 2005, 226, 239-252.
(7) Saleh, N.; Sirk, K.; Liu, Y.; Phenrat, T.; Dufour, B.; Matyjaszewski, K.; Tilton, R. D.; Lowry, G. V. Environmental Engineering Science 2007, 24, 45-57.
(8) Saleh, N.; Phenrat, T.; Sirk, K.; Dufour, B.; Ok, J.; Sarbu, T.; Matyjaszewski, K.; Tilton, R. D.; Lowry, G. V. Nano Letters 2005, 5, 2489-2494.
(9) Stoffelbach, F.; Griffete, N.; Bui, C.; Charleux, B. Chem. Commun. 2008, 4807-4809.
(10) Li, W.; Min, K.; Matyjaszewski, K.; Stoffelbach, F.; Charleux, B. Macromolecules 2008, 41, 6387-6392.
(11) Rieger, J.; Stoffelbach, F.; Bui, C.; Alaimo, D.; Jerome, C.; Charleux, B. Macromolecules 2008, 41, 4065-4068.
(12) Dire, C.; Magnet, S.; Couvreur, L.; Charleux, B. Macromolecules 2009, 42, 95-103.
(13) Lenoir, S.; Pagnoulle, C.; Galleni, M.; Compere, P.; Jerome, R.; Detrembleur, C. Biomacromolecules 2006, 7, 2291-2296.
(14) Thomassin, J.-M.; Lenoir, S.; Riga, J.; Jerome, R.; Detrembleur, C. Biomacromolecules 2007, 8(4), 1171-1177.
(15) Buetuen, V.; Liu, S.; Weaver, J. V. M.; Bories-Azeau, X.; Cai, Y.; Armes, S. P. Reactive & Functional Polymers 2006, 66, 157-165.