End-group transformation chemistry
Advantages:
-
Incorporate functionality incompatible with polymerization
-
Characterization prior to further functionalization
-
Prepare ω- and α,ω-telechelic polymers
-
Block copolymers, immobilized to surfaces, etc.
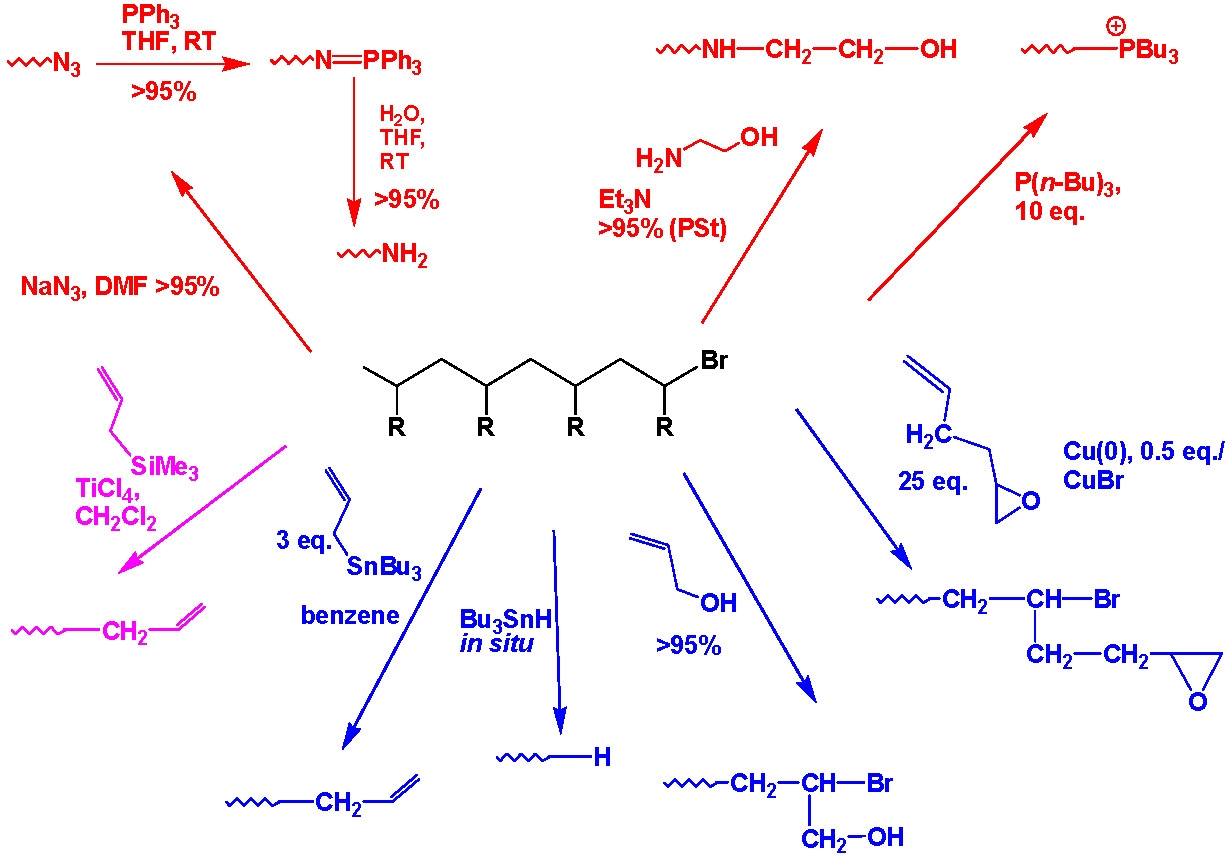
The halide end-functionality, frequently present on the active chain end(s) of polymers prepared by ATRP, particularly polystyrenes or polyacrylates, can participate in nucleophilic substitution reactions. This strategy has been used for the synthesis of a plethora of end-functional well-defined polymeric materials. The advantages are that one can incorporate functionality incompatible with the polymerization process, and if desired the material can be characterized prior to further functionalization. This procedure allows the preparation of ω- and α,ω-telechelic polymers and the selection of functionality suitable for further specific reactions such as attachment to bio-materials or materials that can be immobilized to surfaces, etc.
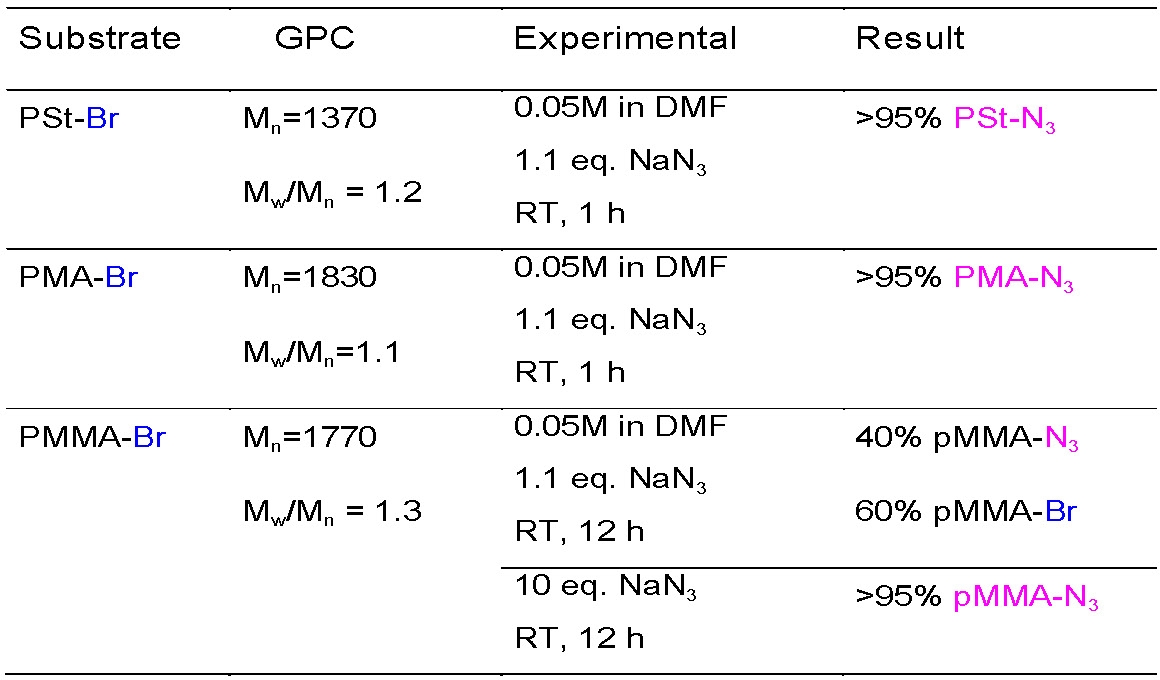
Sample conditions for azidation of the terminal halides on the three major classes of monomers are:
An early example is the reaction of halogen-capped polymers with sodium azide.(1-3) A diazido-terminated polySty prepared in this way could be further reduced with tributylphosphine in THF in the presence of water (moisture) to yield a well-defined diamino-terminated polymer, that, in turn, could be used in a step-growth process with terephthaloyl chloride leading to polyamides with controlled-length polystyrene segments.(2)
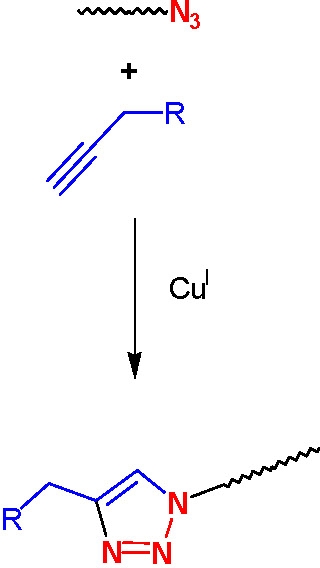
Azido-terminated polymers can also be used in click chemistry modifications with acetylene derivatives to incorporate various functional groups. Polymers with phopshonium salt end-groups were prepared from bromine-terminated polystyrene or polyacrylates, and Bu3P.(4) Mercapto-terminated polystyrene was prepared by the reaction of the corresponding bromo-compound with either thiodimethylformamide or thiourea followed by reaction with a nucleophilic compound.(5)
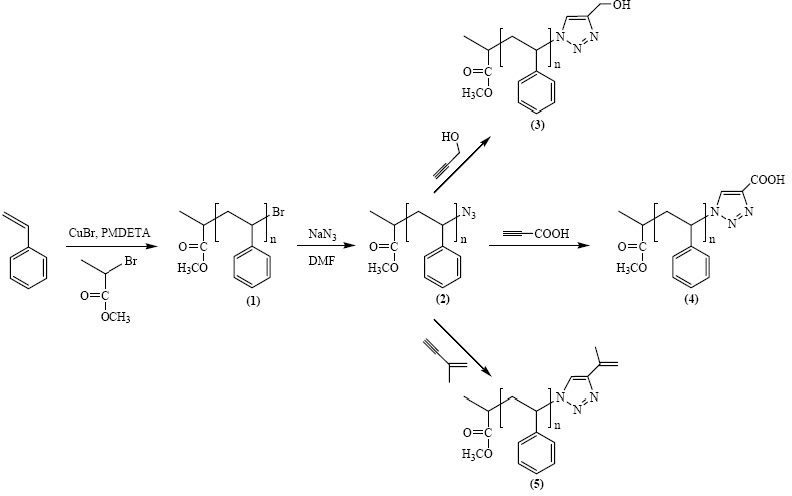
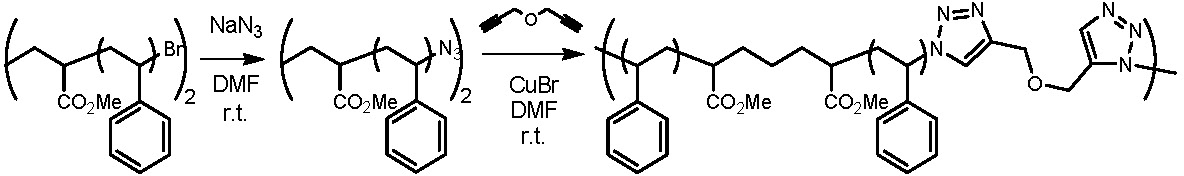
This approach can easily be adapted to provide a step growth coupling reaction from telechelic copolymers prepared by ATRP.(6)
The effect of ligands and solvent on copper-catalyzed click reactions were recently reported, and catalysts based on metals other than copper, such as platinum and palladium were studied.(7)
Many other end-group transformations using polymers prepared by ATRP are described in two extensive review papers.(8,9)
REFERENCES
(1) Matyjaszewski, K.; Nakagawa, Y.; Gaynor, S. G. Macromol. Rapid Commun. 1997, 18, 1057-1066.
(2) Coessens, V.; Nakagawa, Y.; Matyjaszewski, K. Polym. Bull. 1998, 40, 135-142.
(3) Coessens, V.; Matyjaszewski, K. J. Macromol. Sci., Pure Appl. Chem. 1999, A36, 667-679.
(4) Coessens, V.; Matyjaszewski, K. J. Macromol. Sci., Pure Appl. Chem. 1999, A36, 653-666.
(5) Tsarevsky, N. V.; Sumerlin, B. S.; Matyjaszewski, K. Macromolecules 2005, 38, 3558-3561.
(6) Lutz, J.-F.; Boerner, H. G.; Weichenhan, K. Macromolecular Rapid Communications 2005, 26, 514-518.
(7) Golas, P. L.; Tsarevsky, N. V.; Sumerlin, B. S.; Matyjaszewski, K. Macromolecules 2006, 39, 6451-6457.
(8) Coessens, V.; Pintauer, T.; Matyjaszewski, K. Prog. Polym. Sci. 2001, 26, 337-377.
(9) Matyjaszewski, K.; Xia, J. Chem. Rev. 2001, 101, 2921-2990.
