Development of Controlled/"Living" Radical Polymerization
Review Articles on CRP
Factors Influencing the Evolution of CRP (Focusing on ATRP)
Equilibrium for the Three Major CRP processes
Introduction:
The following figure shows that on March 2014 there have been over 25,000 papers published on Controlled Radical Polymerization, (CRP), since 1993 and more than 13,000 on ATRP since 1995. Throughout most of the pages on this web site we will predominately use the term "controlled" to describe the reactions discussed since the objective of the experiments is to "control" one or more properties of the formed polymer and, since we are discussing radical process and some level of termination reactions are inevitable, the term "living" is not strictly true.
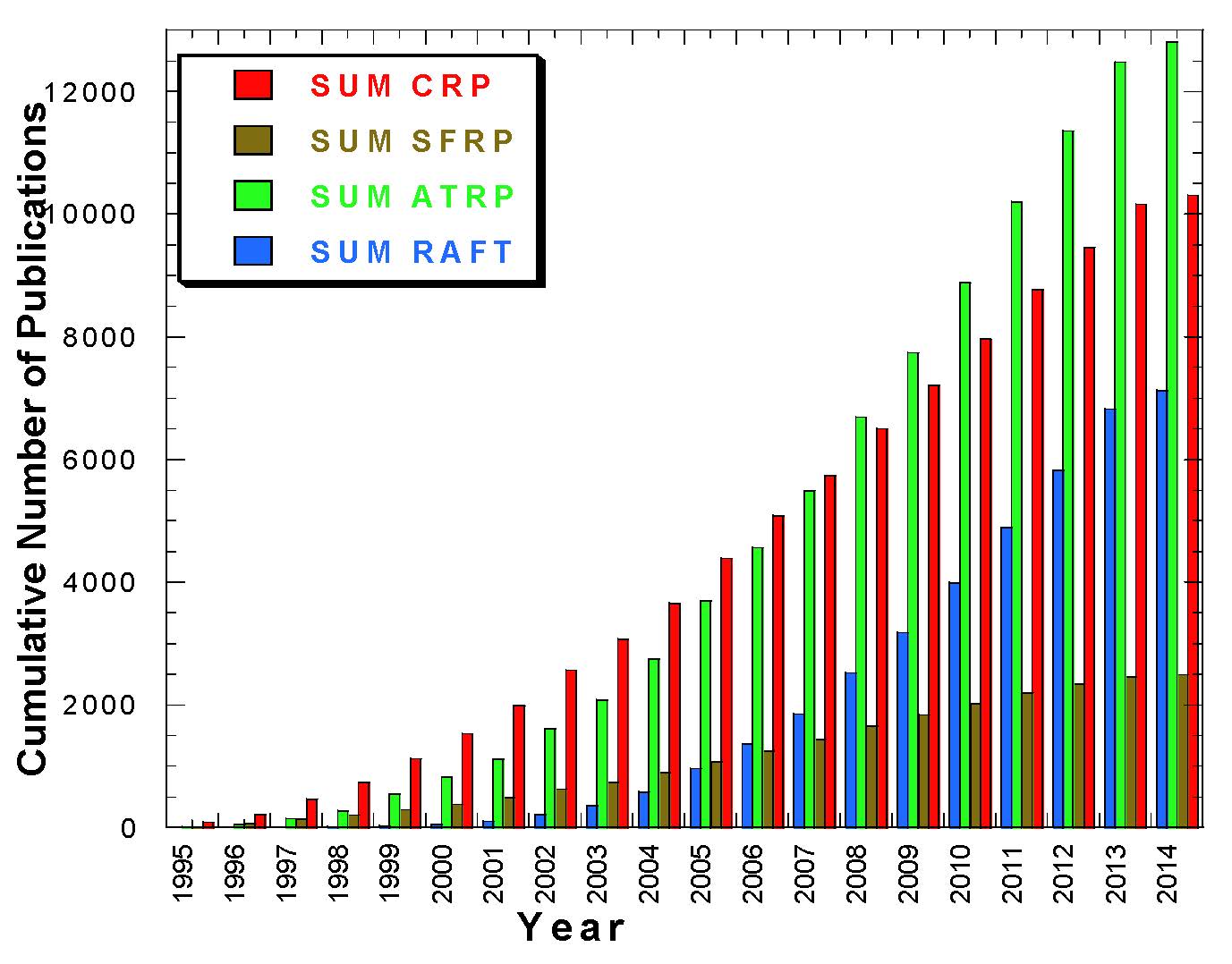
The data displayed in the figure above is current to March 2014 and was obtained by conducting a search on SciFinder Scholar using the following terms: "controlled radical polymn" or "living radical polymn" (‘SUM CRP' in the figure), "ATRP or atom transfer (radical) polymn" (i.e. SUM ATRP in the figure refers to ATRP only, this does not include terms like metal mediated or metal catalyzed radical polymerization), "NMP or SFRP or nitroxide mediated polymn or stable free polymn" (‘SUM SFRP') and "RAFT or reversible addition transfer or degenerative transfer or catalytic chain transfer" (‘SUM DT'). The latter two terms were refined with a term "radical polymn" since they coincide with other popular abbreviations used for N-methylpyrrolidone or raft-associated proteins.
In addition to the growth in publications shown above there has been a corresponding continuous growing interest in protecting various aspects of materials prepared by ATRP. There have been close to 3000 published US patents and US patent applications (much duplication) utilizing the term ATRP within the text of the application for the preparation of materials targeting specific applications in the same time frame. The reason for this explosion of interest in intellectual property is an expanding market for specialty functional materials prepared from the wide range of readily available radically (co)polymerizable monomers.(1)Applications include coatings, adhesives, surfactants, dispersants, lubricants, gels, additives, novel thermoplastic elastomers, electronics, health and beauty products, biomaterials, drug delivery systems, in fact almost any market requiring a material with a specific set of well defined properties.
Furthermore with the development of new ATRP systems that require very low concentrations of catalyst it is now possible to use standard FRP industrial equipment for a controlled synthesis of materials containing one or more segments prepared by ATRP.(2) A similar statement can now be made for emulsion polymerization with the development of a true ab initio emulsion ATRP(3) and with the identification of conditions for conducting an ARGET ATRP in aqueous media allowing direct synthesis of a well-defined protein-polymer hybrid by the "grafting from" method.(4)
There is a lot of overlap between the terms but the figure does show that there has been a continuous increase in the level of interest to use the various controlled polymerization procedures for the preparation of functional materials.
Review Articles on CRP:
There have been five ACS Symposia on CRP in the past decade. Proceedings from the symposia held in 1997, 1999, 2002, 2005 published as single ACS Symposium series.(5-8) The fifth symposium in the series, which was held at the ACS Meeting in Philadelphia on August 17-21, 2008, was published in two volumes in August of 2009(9) one covering progress in ATRP, ACS Symposium Series 1023, and the other progress in RAFT, ITP, NMP and OMRP, ACS Symposium Series 1024. A sixth meeting on Controlled Radical Polymerization resulted in two additional ACS Symposium Series, 1100 and 1101; one discussing Mechanisms and Techniques and the other Materials and Applications that were edited in conjunction with former group members Brent Sumerlin and Nick Tsarevsky in which Kris provided an introduction to CRP.(10)Several extensive reviews and book chapters on each of the three recently developed, more broadly applied Controlled/Living Radical Polymerization (CRP) processes have also been published(11-24) with a recent review discussing all CRP processes providing a balanced perspective.(25) The current status and future perspectives of ATRP have also been reviewed with special emphasis on mechanistic understanding of ATRP, recent synthetic and process development, and new controlled polymer architectures enabled by ATRP.(26) Indeed another review provided a discussion of ATRP and mechanistically similar atom transfer radical addition (ATRA) in which basic phenomenology, components, and applications are reviewed.(27)
Factors Influencing the Evolution of CRP (Focusing on ATRP):
The development of CRP, and even more specifically Atom Transfer Radical Polymerization (ATRP), is based on understanding and integration of chemistry developed over the past 70 years in the fields of organic chemistry, coordination chemistry, conventional radical polymerization, and living ionic polymerizations, augmented by computational, electrochemistry and inorganic chemistry that came together in the mid-1990’s.(28-43)
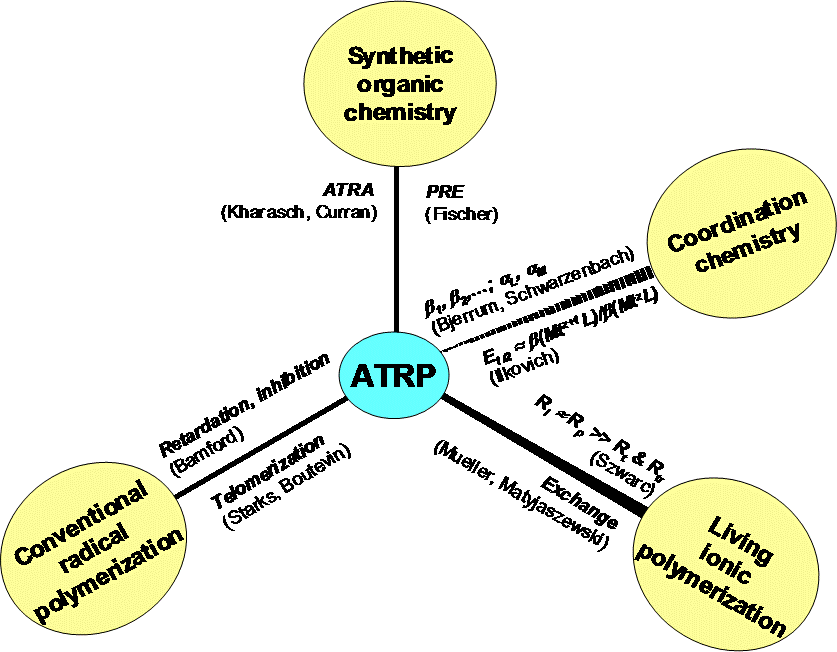
There are several CRP processes that have been developed based on this fundamental understanding, but the systems that are presently receiving the most attention are Atom Transfer Radical Polymerization, (ATRP),(44-45) which is based on the fundamental work on ATRA(27-30,46) and coordination chemistry:(31-36) Stable Free Radical Polymerization (SFRP)(47) including Nitroxide Mediated Polymerization (NMP) (21,48-51) and OrganoMetalic Radical Polymerization (OMRP) or transition metal mediated radical polymerization,(52-53) and Degenerative Transfer (DT)(54-56) processes including Reversible Addition Fragmentation Transfer (RAFT)(57-59) and Macromolecular Design via the Interchange of Xanthates (MADIX).(60-61)
A review of the kinetics of CRP has recently been provided by Fukuda.(62)
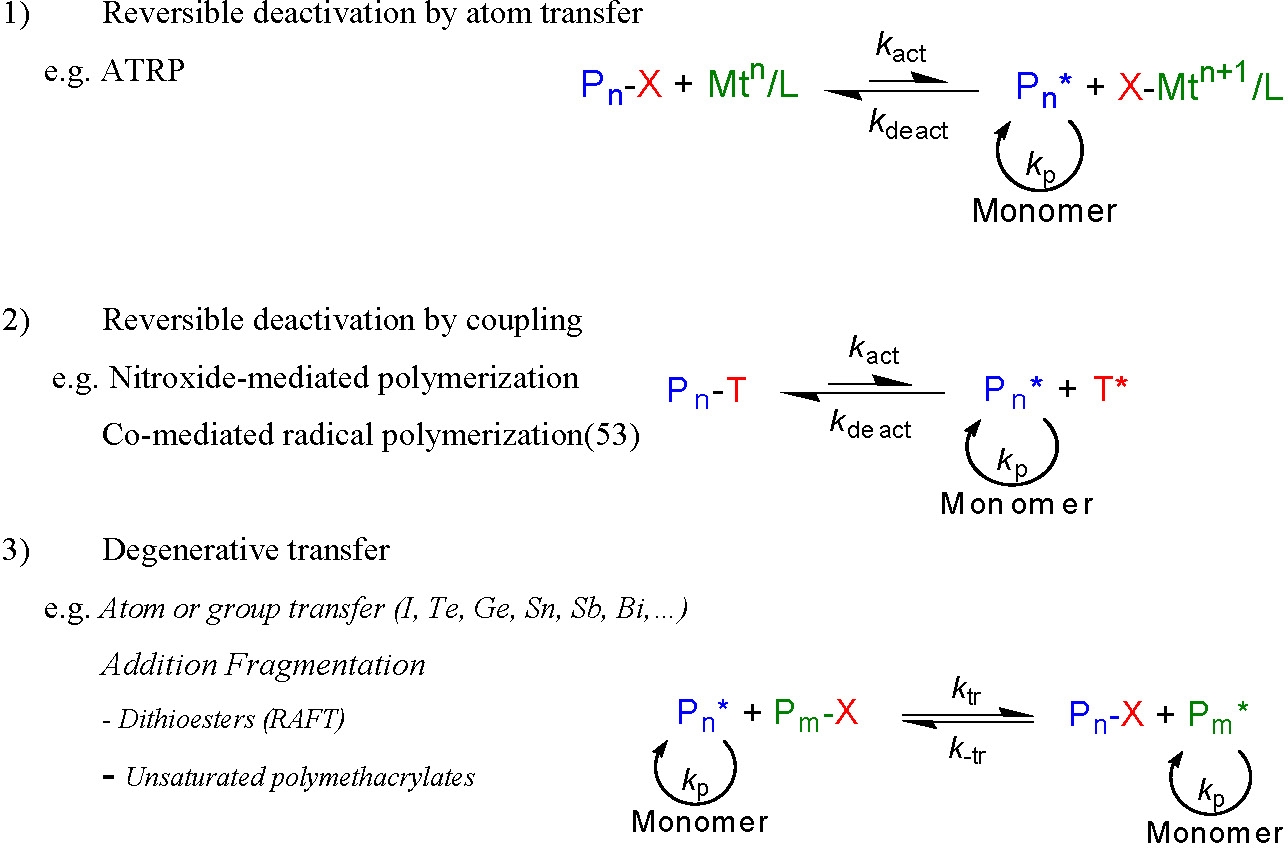
Discussion of the Required Equilibrium Conditions for each of the Three Major CRP Processes:
The dynamic equilibrium required for control over each of the three major CRP processes is shown below.
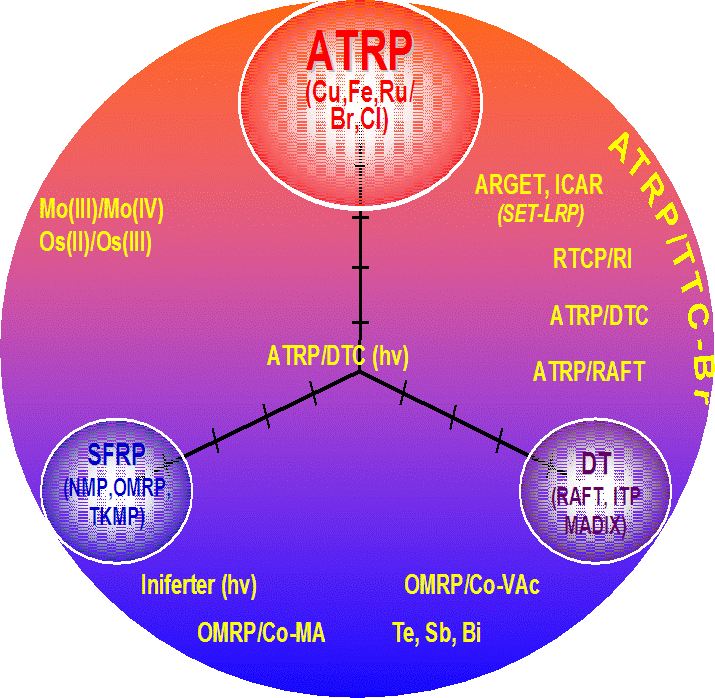
Recent advances in all of the CRP procedures have reduced the clear differences that once existed between these three widely applied means of controlling a radical based polymerization and the following figure is one way of viewing this merging, or overlapping of CRP procedures. The center of the diagram is occupied by a procedure abbreviated as ATRP/DCT (hv) which is part of the most recent addition to page 4 of this web site where a section describing ATRP conducted in the presence Initiators containing Radically Transferable Groups is detailed.
In the case of "classic" ATRP and NMP, which obey the persistent radical effect (PRE),(32) control is attained by:
- extending, or fragmenting, the life of propagating chains during the reaction from <1s to >1 h
- enabling quantitative (and close to simultaneous) initiation of each polymer chain, i.e. going from Ri << Rp as is the case for conventional RP to Ri >> Rp for CRP
- providing conditions whereby the molecular weight of the final polymer is determined by the ratio of polymerized monomer to added initiator, (DPn = Δ[M]/[I])
- selection of comonomers and initiator that allows control over tele-functionality, composition, and topology
- all of these objectives are accomplished by formation of a dynamic equilibrium between a low concentration of propagating radicals and an excess of dormant species.
The work of the Matyjaszewski group is summarized within this web page.
As noted above, in the case of an ATRP reaction the appropriate selection of suitable catalyst complexes that allows an ATRP to be conducted in a variety of reaction media is based not only on the fundamental work on transition metal catalyzed ATRA, or “Kharasch,” reactions, but also on understanding redox properties(33-34) and stability constants of transition metal complexes.(35) Together they provide the tools for catalyst selection summarized in the “Fundamentals of ATRP” and detailed in the “Mechanism and Catalyst Development” sections of this web page.
REFERENCES
(1) Matyjaszewski, K.; Spanswick, J. Materials Today 2005, 8, 26-33.(2) Jakubowski, W.; Spanswick, J.; Mueller, L.; Matyjaszewski, K. In PCT Int. Appl.; (Carnegie Mellon University, USA). WO 2007075817, 2007; p 65pp.
(3) Min, K.; Gao, H.; Matyjaszewski, K. Journal of the American Chemical Society 2006, 128, 10521-10526.
(4) Simakova, A.; Averick, S. E.; Konkolewicz, D.; Matyjaszewski, K. Macromolecules 2012, 45, 6371-6379.
(5) Matyjaszewski, K.; Editor. Controlled Radical Polymerization; ACS Symp. Ser. 685: held 13-17 April 1997, in San Francisco, 1998.
(6) Matyjaszewski, K.; Editor. Controlled/Living Radical Polymerization. Progress in ATRP, NMP, and RAFT; ACS Symp. Ser.768: New Orleans, 22-24 August 1999, , 2000.
(7) Matyjaszewski, K.; Editor. Advances in Controlled/Living Radical Polymerization; ACS Symposium Series 854: Boston, 2003.
(8) Matyjaszewski, K.; Editor. Controlled/Living Radical Polymerization; ACS Symposium Series 944: Washington, DC August 27 to September 1, 2005, 2006.
(9) Matyjaszewski, K.; Editor. Controlled Living/Radical Polymerization; ACS Symposium on CRP: Philadelphia August 17-21, 2008 2009; Vol. 1023 and 1024.
(10) Matyjaszewski, K. ACS Symp. Ser. 2012, 1100, 1-13.
(11) Wang, J.-S.; Matyjaszewski, K. Macromolecules 1995, 28, 7572-7573.
(12) Davis, K. A.; Matyjaszewski, K. Statistical, Gradient and Segmented Copolymers by Controlled/Living Radical Polymerizations; Springer Verlag: BERLIN, 2002.
(13) Matyjaszewski, K.; Xia, J. Chem. Rev. 2001, 101, 2921-2990.
(14) Kamigaito, M.; Ando, T.; Sawamoto, M. Chem. Rev. 2001, 101, 3689-3745.
(15) Tsarevsky, N. V.; Matyjaszewski, K. Chem. Rev. 2007, 107, 2270-2299.
(16) Hawker, C. J.; Bosman, A. W.; Harth, E. Chem. Rev. 2001, 101, 3661-3688.
(17) Barner-Kowollik, C.; Davis, T. P.; Heuts, J. P. A.; Stenzel, M. H.; Vana, P.; Whittaker, M. Journal of Polymer Science, Part A: Polymer Chemistry 2003, 41, 365-375.
(18) Perrier, S.; Takolpuckdee, P. Journal of Polymer Science, Part A: Polymer Chemistry 2005, 43, 5347-5393.
(19) Moad, G.; Rizzardo, E.; Thang, S. H. Australian Journal of Chemistry 2005, 58, 379-410.
(20) Moad, G.; Rizzardo, E.; Thang, S. H. Australian Journal of Chemistry 2006, 59, 669-692.
(21) Sciannamea, V.; Jerome, R.; Detrembleur, C. Chemical Reviews 2008, 108, 1104-1126.
(22) Zetterlund, P. B.; Kagawa, Y.; Okubo, M. Chemical Reviews 2008, 108, 3747-3794.
(23) Cunningham, M. F. Progress in Polymer Science 2008, 33, 365-398.
(24) Moad, G.; Rizzardo, E.; Thang, S. H. Polymer 2008, 49, 1079-1131.
(25) Braunecker, W. A.; Matyjaszewski, K. Progress in Polymer Science 2007, 32, 93-146.
(26) Matyjaszewski, K. Macromolecules 2012, 45, 4015-4039.
(27) Pintauer, T.; Matyjaszewski, K. Encycl. Radicals Chem., Biol. Mater. 2012, 4, 1851-1894.
(28) Kharasch, M. S.; Kuderna, B. M.; Urry, W. H. Journal of Organic Chemistry 1948, 13, 895-902.
(29) Curran, D. P. Synthesis 1988, 489-513.
(30) Minisci, F.; Fontana, F.; Araneo, S.; Recupero, F.; Banfi, S.; Quici, S. Journal of the American Chemical Society 1995, 117, 226-232.
(31) Fischer, H. Journal of the American Chemical Society 1986, 108, 3925-3927.
(32) Fischer, H. Chemical Reviews 2001, 101, 3581-3610.
(33) Lingane, J. J. Chem. Rev. 1941, 29, 1-35.
(34) Rorabacher David, B. Chem Rev 2004, 104, 651-697.
(35) Vlcek, A. A. Coordination Chemistry Reviews 1982, 43, 39-62.
(36) Kochi, J. K. Science 1967, 155, 415-424.
(37) Bjerrum, J. Metal Ammine Formation in Aqueous Solution. Theory of the Reversible Step Reactions, 1957.
(38) Szwarc, M. Nature 1956, 178, 1168-1169.
(39) Matyjaszewski, K.; Lin, C. H. Makromolekulare Chemie, Macromolecular Symposia 1991, 47, 221-237.
(40) Litvinenko, G.; Mueller, A. H. E. Macromolecules 1997, 30, 1253-1266.
(41) Otsu, T. J. Polym. Sci., Part A: Polym. Chem. 2000, 38, 2121-2136.
(42) Boutevin, B. J. Polym. Sci., Part A: Polym. Chem. 2000, 38, 3235-3243.
(43) Bamford, C. H. Redox Initiators; vol. 3 of Comprehensive Polymer Science, Permagon Press, 1992.
(44) Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Macromolecules 1995, 28, 1721-1723.
(45) Wang, J.-S.; Matyjaszewski, K. Journal of the American Chemical Society 1995, 117, 5614-5615.
(46) Pintauer, T. Eur. J. Inorg. Chem. 2010, 2449-2460.
(47) Georges, M. K.; Veregin, R. P. N.; Kazmaier, P. M.; Hamer, G. K. Macromolecules 1993, 26, 2987-2988.
(48) Hawker, C. J.; Bosman, A. W.; Harth, E. Chemical Reviews 2001, 101, 3661-3688.
(49) Studer, A.; Schulte, T. Chemical Record 2005, 5, 27-35.
(50) Grubbs, R. B. Polym. Rev. 2011, 51, 104-137.
(51) Tebben, L.; Studer, A. Angew. Chem., Int. Ed. 2011, 50, 5034-5068.
(52) Poli, R. Angewandte Chemie, International Edition 2006, 45, 5058-5070.
(53) Hurtgen, M.; Detrembleur, C.; Jerome, C.; Debuigne, A. Polym. Rev. 2011, 51, 188-213.
(54) Matyjaszewski, K.; Gaynor, S.; Wang, J.-S. Macromolecules 1995, 28, 2093-2095.
(55) Moad, C. L.; Moad, G.; Rizzardo, E.; Thang, S. H. Macromolecules 1996, 29, 7717-7726.
(56) Yamago, S. Chem. Rev. 2009, 109, 5051-5068.
(57) Rizzardo, E.; Chiefari, J.; Mayadunne, R.; Moad, G.; Thang, S. Macromolecular Symposia 2001, 174, 209-212.
(58) Destarac, M.; Charmot, D.; Franck, X.; Zard, S. Z. Macromol. Rapid Commun. 2000, 21, 1035-1039.
(59) Barner-Kowollik, C.; Blinco, J. P.; Destarac, M.; Thurecht, K. J.; Perrier, S. Encycl. Radicals Chem., Biol. Mater. 2012, 4, 1895-1929.
(60) Destarac, M.; Brochon, C.; Catala, J.-M.; Wilczewska, A.; Zard, S. Z. Macromolecular Chemistry and Physics 2002, 203, 2281-2289.
(61) Quiclet-Sire, B.; Zard, S. Z. Topics in Current Chemistry 2006, 264, 201-236.
(62) Goto, A.; Fukuda, T. Progress in Polymer Science 2004, 29, 329-385.
(63) Wayland, B. B.; Peng, C.-H.; Fu, X.; Lu, Z.; Fryd, M. Macromolecules 2006, 39, 8219-8222.
