Use of functional ATRP initiators
Advantages:
-
Direct functionalization
-
No post-polymerization modification
-
Yields telechelic polymers with preselected α-functionality
-
Applicable to multiple protected and unprotected functionalities
A wide variety of α-functionalized ATRP initiators have been used to prepare hetero-telechelic polymers. A few examples of functional initiators used in styrene polymerization are shown below and a more extensive listing is provided in the review paper by Coessens et al.(1)
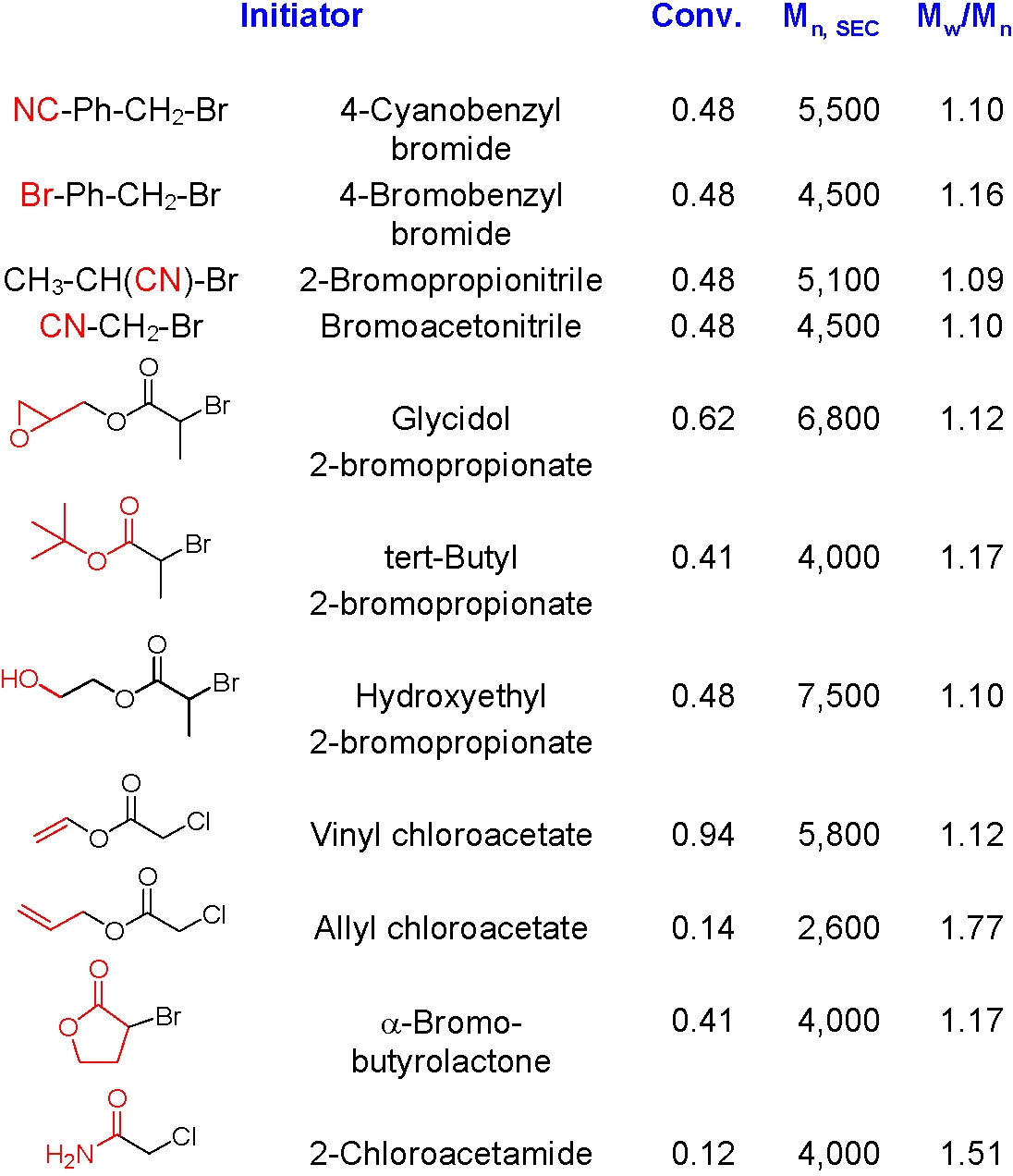
A more recent review includes several additional α-functional groups. (2)
One route to α,ω-homo-telechelic copolymers is to couple a copolymer prepared using a mono-functional initiator in an atom transfer radical coupling reaction (ATRC). The atom transfer coupling reaction can be driven to completion by continuous removal of the deactivator from the reaction medium. Initially this was accomplished by the addition of a transition metal in the zero oxidation state.(3,4) However, a more environmentally benign approach was recently developed using ascorbic acid or reducing sugars to drive the coupling reaction.
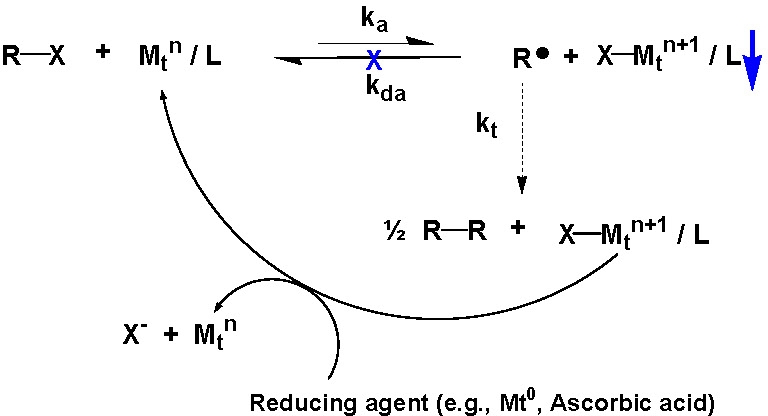
The coupling efficiency of (meth)acrylates can be improved by the addition of one mole of styrene immediately prior to the addition of the coupling agent.(3,5,6)
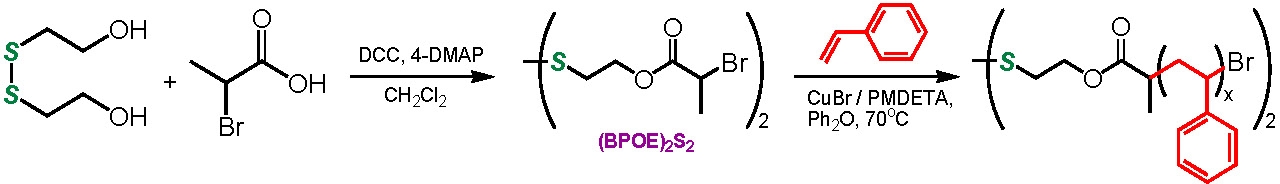
Difunctional initiators can also be employed to introduce functionality into the mid point of a chain and when coupled with the coupling reaction can lead to the formation of degradable polymers that degrade to oligo/polymeric fragments of known molecular weight and narrow PDI. This was exemplified recently by the use of an initiator with an internal disulfide bond to polymerize styrene. The disulfide bond could degrade under a reducing environment to yield the corresponding thiol-terminated polystyrene. The thiol end groups were efficiently coupled back to the starting disulfide by oxidation with FeCl3.(7) Polar monomers could also be polymerized using the same difunctional initiator.(8) The implication of this incorporation of a degradable link into the backbone of a polymer will be discussed fully in the section discussing degradable networks.
Another approach to coupling is addition of a non-polymerizable monomer, such as an a-a disubstituted olefin, to the later stages of an ATRP reaction and how an understanding of this process can lead to a novel catalytic coupling process.(9) The α-α disubstituted monomer can be exemplified by an α-methyl styrene unit. When this new α-methylstyryl radical end group, formed by addition of the α-methyl styrene monomer to an oligomer or polymer radical chain end, receives back from the transition metal complex, in the reverse redox reaction, the initially transferred radically transferable atom or group a new polymer end group, containing both an α-methyl- and an α-bromo- group is formed. This type of end group, containing an α-substituant, an α-bromo- group and a β-hydrogen, can lose hydrogen bromide, forming a new functional exo-olefinic bond. The formed macromonomer can then repeat the same step when incorporated into a second chain. This can be viewed as a catalytic coupling reaction. (10)
REFERENCES
(1) Coessens, V.; Pintauer, T.; Matyjaszewski, K. Prog. Polym. Sci. 2001, 26, 337-377.
(2) Matyjaszewski, K.; Tsarevsky, N. V. Nature Chemistry 2009, 1, 276-288.
(3) Sarbu, T.; Lin, K.-Y.; Spanswick, J.; Gil, R. R.; Siegwart, D. J.; Matyjaszewski, K. Macromolecules 2004, 37, 9694-9700.
(4) Otazaghine, B.; David, G.; Boutevin, B.; Robin, J. J.; Matyjaszewski, K. Macromolecular Chemistry and Physics 2004, 205, 154-164.
(5) Otazaghine, B.; Boutevin, B. Macromolecular Chemistry and Physics 2004, 205, 2002-2011.
(6) Matyjaszewski, K.; Gaynor, S. G.; Coca, S. In PCT Int. Appl.; (Carnegie Mellon University, USA). WO 9840415, 1998; p 230 pp.
(7) Tsarevsky, N. V.; Matyjaszewski, K. Macromolecules 2002, 35, 9009-9014.
(8) Tsarevsky, N. V.; Matyjaszewski, K. Macromolecules 2005, 38, 3087-3092.
(9) Matyjaszewski, K.; Gaynor, S. G.; Paik, H.-j.; Pintauer, T.; Pyun, J.; Qiu, J.; Teodorescu, M.; Xia, J.; Zhang, X. In PCT Int. Appl.; (Carnegie Mellon University, USA). WO 0056795, 2000; p 200 pp.
(10) Shim, A. K.; Coessens, V.; Pintauer, T.; Gaynor, S.; Matyjaszewski, K. Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.) 1999, 40, 456-457.
