Ligands
A list of the ligands most frequently employed by members of the group over the past fifteen years for ATRP reactions are listed below together with the abreviations used in this series of :Starting Points".
bpy: 2,2'-bipyridine
dNbpy: 4,4'-Di-5-nonyl-2,2'-bipyridine (It is not commercially available. A synthesis procedure is provided below however the group presently uses 4,4-dinonyl-2,2'-dipyridyl from Aldrich)
tNtpy: 4,4',4''-tris(5-nonyl)-2,2':6',2''-terpyridine
PMDETA: N,N,N',N',N''-Pentamethyldiethylenetriamine
HMTETA: 1,1,4,7,10,10-Hexamethyltriethylenetetramine
Me6TREN: Tris(2-dimethylaminoethyl)aminea
BPMODA: N,N-bis(2-pyridylmethyl)octadecylamine
TPEDA: N,N,N',N'-tetra[(2-pyridal)methyl]ethylenediamine
TPMA: tris[(2-pyridyl)methyl]aminea
TREN: tris(2-aminoethyl)amine was obtained from Aldrich
BA6TREN: tris(2-bis(3-butoxy-3-oxopropyl)aminoethyl)amine , and
EHA6TREN: tris(2-bis(3-(2-ethylhexoxy)-3-oxopropyl)aminoethyl)amine were synthesized according to published procedures.(1,2)
LA6TREN: Tris(2-bis(3-dodecoxy-3-oxopropyl)aminoethyl)amine was prepared, via a slightly modified procedure in a reported work, by further extending the reaction at 50 oC for another 24 hours.
a Now available in laboratory quantities from ATRP Solutions (http://atrpsolutions.com/includes/PB_3%20Ligands.pdf)
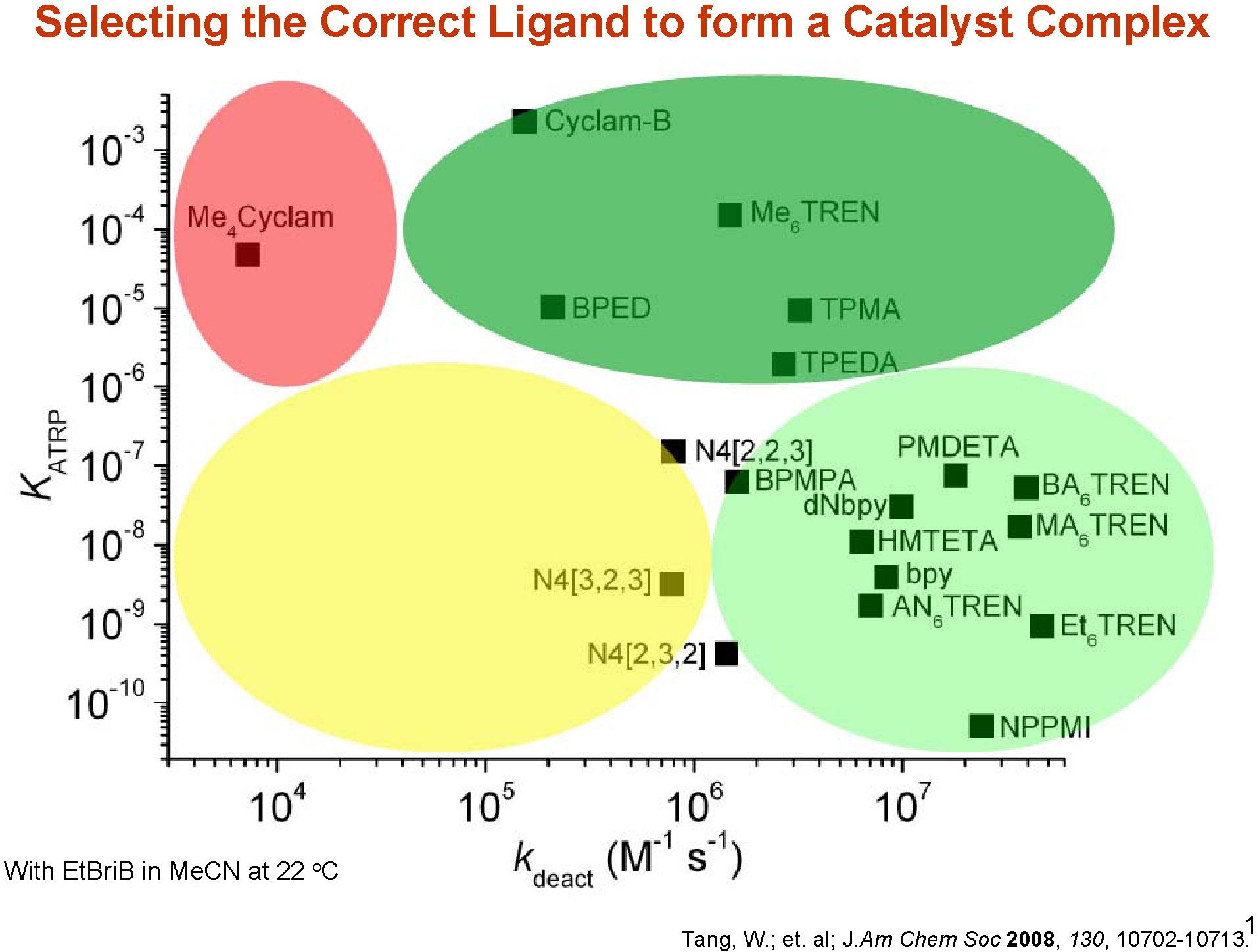
The ligands used most frequently for low, ppm, catalyst reactions are Me6TREN and TPMA. As shown in the following figure, one of the primary reasons for this selection is that cataltst complexes formd with these ligands display high values for kdeact in addition to higher kATRP.
Synthesis of 4,4'-Bis(5-nonyl)-2,2'-bipyridine (dNbpy)
250 mL of 4-(1-butyl-pentyl)pyridine and 10 g of Pd/C were placed in a 500 mL flask, and heated to 210 °C in an oil bath under argon with reflux and rapid stirring. After 1 week, the reaction mixture was cooled, filtered first through filter paper (Pd/C was rinsed with Et2O), then filtered over celite. The ether was removed by rotoevaporation, and the remaining yellow/beige liquid was vacuum distilled to remove the unreacted pyridine. The residue was then distilled under high vacuum; 41 g of 99 % pure dNbpy was collected at 180 °C under 10-7 Torr, which slowly started to crystallize upon cooling.
REFERENCES
1) Zeng, F.; Shen, Y.; Zhu, S.; Pelton, R. Macromolecules 2000, 33, 1628-1635.
2) Gromada, J.; Spanswick, J.; Matyjaszewski, K. Macromolecular Chemistry and Physics 2004, 205, 551-566.
