ATRP of Acrylates
The solubility of different poly(acrylates) should be taken into consideration when selecting a solvent for the reaction. In the following series of examples different solvents are indicated.
Methyl Acrylate
n-Butyl Acrylate
-
Difunctional Macroinitiator
-
ARGET ATRP of n-Butyl Acrylate
-
From a Polyethylene Multifunctional Macroinitiator
-
From the Surface of a Silicon Wafer
-
From Functional Colloids
-
From Initiator-coated Silica Particles in Miniemulsion
-
Molecular Brushes via AGET ATRP in Miniemulsion
-
Development of an ab-initio Emulsion ATRP of BA
-
Block Copolymer in an ab-initio Emulsion Polymerization
Poly(t-Butyl Acrylate)
Lauryl Acrylate
2-Hydroxyethyl Acrylate
Glycidyl Acrylate
Materials:
n-Butyl acrylate (BA, Aldrich), 2-ethylhexyl acrylate (EHA, Aldrich) and lauryl acrylate (LA, Aldrich) were purified by passing the monomer through a column filled with basic aluminum oxide (Aldrich) to remove the antioxidant/inhibitor. The monomers were stored at -5 oC for later use.
ATRP of Methyl Acrylate
The experimental procedures used for the following polymerizations are fundamentally the same as those detailed for styrene.MA : EBiB : CuBr : PMDETA = 200 : 1 : 0.5 (anisole as solvent) at 60 oC for 220 min;
Mn = 10,200; Mw/Mn = 1.07.
N,N,N',N'-tetra[(2-pyridal)methyl]ethylenediamine (TPEDA) as Ligand
The ATRP of MA using ethyl 2-bromoisobutyrate (EBiB) as initiator, 10 mol % of CuBr/TPEDA relative to EBiB as catalyst, in bulk at 80 oC, was very fast (98% conv. in 1.5 h) and yielded PMA with low PDI, 1.13.
M:I:Cu:L = 116:1:0.1:0.1, Mn = 9,700; Mw/Mn = 1.13.
Bulk Polymerization of Methyl Acrylate/Methyl 2-Bromopropionate/PMDETA
As a representative example, a 10 mL Schlenk flask was charged with Cu0 (2 mg; 0.032 mmol), Cu(OTf)2 (12 mg; 0.033 mmol), N,N,N',N'',N''- pentamethyldiethylenetriamine (14 μL; 0.067 mmol), methyl 2-bromopropionate (37 μL; 0.33 mmol), and methyl acrylate (6 mL; 66.6 mmol) followed by 0.6 mL of chlorobenzene as an internal reference. The reaction flask was charged with a stir bar and then fitted with a rubber septum. The reaction solution was then put through freeze-vacuum-thaw cycles three times to remove dissolved gases and then put under an argon atmosphere. The flask was then immersed in an oil bath held by a thermostat at 80 °C with rigorous stirring. A homogeneous blue solution was observed. At various times, samples were taken via syringe and diluted with THF. The volume lost by sample removal was replaced with argon. The samples were used to monitor percent monomer conversion relative to the internal reference (GC) and molecular weight (SEC). After 60 min, 75% monomer conversion was observed. (Mn = 12,900; Mw/Mn = 1.16)
ATRP of n-Butyl Acrylate
For actual DP ~ 300
MBrP - 64 ml (0.536 mmol) : CuBr - 0.0780 g (0.536 mmol) : PMDETA - 112 μL (0.536 mmol) : BA - 30 ml (0.26 mole) plus anisole 5 ml. T = 70 oC
All liquid reagents were put in a Schlenk flask, the solution was degassed by three freeze-pump-thaw cycles, the flask with the frozen liquids was filled with nitrogen, CuBr was added, and several times the flask was evacuated followed by filling with nitrogen (the mixture was still frozen). Then the flask was placed in an oil bath at 70 oC. After 21 hours (conversion 81.7% based on GC), the polymer was isolated and purified by passing a THF solution of the product through a column filled with alumina. The molecular weight of the macroinitiator, after removing the unreacted BA by drying in vacuum at 70 oC to a constant weight was determined by GPC to be 32,290; Mw/Mn = 1.17 (THF as the eluent, polystyrene calibration) or 44,220; Mw/Mn = 1.13 (DMF as the eluent, polystyrene standards).
For DP ~ 120:
The targeted MW here was lower than in the previous experiment therefore a higher ratio of catalyst to initiator was used, 1:2.5 instead of the previous ratio of 1:1, in order to slow down the reaction.
MBrP - 40 ml (0.335 mmol) : CuBr - 0.0193 g (0.134 mmol) : PMDETA - 28 μL (0.134 mmol) " BA - 10 ml and anisole 4 ml. T = 70 oC
The same procedure as the one detailed above was used. The reaction was stopped after 13 hours (conversion 70.7% based on GC). The polymer was isolated and purified as in the previous case. The molecular weight of the macroinitiator determined by GPC was 14,650 and Mw/Mn = 1.20 (THF as the eluent, polystyrene calibration) or 18,850 and Mw/Mn = 1.11 (DMF as the eluent, polystyrene standards).
Preparation of a Poly(butyl acrylate) Difunctional Macroinitiator
Ratio of Reagents: 150:1:0.5:0.5
Degassed butyl acrylate (20 mL) was added to a reaction flask, followed by degassed anisole (2 mL). The solution was then degassed for several minutes. The initiator (dibromo dimethyl heptanedioate, 101 μL) was then added, followed by PMDETA (48.5 μL) and CuBr (33.4 mg). The solution was degassed for several minutes after each addition was made. The reaction mixture was then lowered into a 70°C oil bath. The reaction was monitored by GC (with anisole as the internal standard). The conversion was fairly linear with time, and the reaction was stopped after 215 minutes (final conversion 50%).
Synthesis of Difunctional P(n-butyl acrylate) Macroinitiator for Preparation of a Thermoplastic Elastomer (MW~50,000):
(Richard, R. E.; Schwarz, M.; Ranade, S.; Chan, A. K.; Matyjaszewski, K.; Sumerlin, B. Biomacromolecules 2005, 6, 3410-3418.)
CuBr (0.233 g, 1.62 mmol) was added to a 500-mL Schlenk flask. The flask was purged by repetitions of vacuum and nitrogen filling (x3). Nitrogen-purged BA (125 g, 975 mmol) and anisole (14 mL) were added via syringe under a nitrogen atmosphere. The resulting solution was purged for an additional 15 minutes by bubbling with nitrogen. PMDETA (0.34 μL, 1.63 mmol) was added by syringe followed by purging with nitrogen for 35 min. Dimethyl 2,6-dibromoheptanedioate (0.36 mL, 1.63 mmol) was via syringe and the sealed flask was placed in a preheated oil bath at 70 oC. Samples were taken periodically and after 8 h, when conversion determined by GC was 77% the flask was removed from the heat and opened to air overnight to ensure complete oxidation of the catalyst. The polymerization solution was diluted with THF and passed through an alumina (activated neutral) column to remove the catalyst. The excess solvent was removed by rotary evaporation, and the remaining material was dried under high vacuum for 48 h. MW determined by GPC with PS calibration was 49,000 vs. 59,000 theoretical with Mw/Mn = 1.23.
Synthesis of PBA-based Difunctional Macroinitiator:
(Kowalewski, T.; Tsarevsky, N. V.; Matyjaszewski, K. Journal of the American Chemical Society 2002, 124, 10632-10633.)
3.13 x 10-2 g (1.8 x 10-4 mol) PMDETA was dissolved in 20 ml (17.88 g, 0.14mol) BA and 1 ml of anisole (internal standard for GC) in a Schlenk flask. The mixture was then degassed by four freeze-pump-thaw cycles, the flask was filled with nitrogen,and 2.55 x 10-2 g (1.8x10-4 mol) of CuBr was added while the mixture was still frozen. The flask was sealed and air was removed by evacuating the flask and back-filling with nitrogen several times. After thawing the mixture, the reaction flask was immersed in an oil-bath heated to 70 oC, and the initiator DM-2,6-DBHD (3.8x10-2 ml, 6.04x10-2 g, 1.8x10-4 mol) was added through the side arm. After 16 h, the product was dilluted 200 ml of THF, and the solution was passed through a column filled with alumina to remove the catalyst. Then the solvent was removed and the PBA was dried under vacuum at 60 oC to constant weight. The molecular weight, based on PBA standards, was Mn = 67,600; Mw/Mn = 1.16.
ARGET ATRP of n-Butyl Acrylate with 150 ppm Cu:
(Jakubowski, W.; Matyjaszewski, K. Angewandte Chemie, International Edition 2006, 45, 4482-4486.)
The ratio of BA/EBiB/Cu(II)/tri(2-pyridylmethyl)amine (TPMA)/Sn(EH)2 was 156/1/0.01(50 ppm)/0.03/0.05. The reaction was carried out in anisole 0.2 vol. equiv. vs. monomer at 60 oC. The GPC traces were monomodal with molecular weight close to theoretical values and low Mw/Mn =1.15.
ARGET ATRP of n-Butyl Acrylate with 50 ppm Cu:
The general procedure for ARGET ATRP of nBA, (targeting number average degree of polymerization (DPn) of 160), with 50 ppm of Cu. Degassed nBA (5.0 ml, 35 mmol) and anisole (0.5 ml) were transferred via degassed syringes to a dry, thoroughly purged by flushing with nitrogen, Schlenk flask. Next, CuCl2 complex (0.24 mg, 0.18 x 10-2 mmol)/Me6TREN (0.51 microliters, 0.18 x 10-2 mmol)) in degassed anisole (0.5 ml) was added. The resulting mixture was stirred for 10 minutes and then a purged solution of Sn(EH)2 (7.29 ml, 2.2 x 10-2 mmol) and Me6TREN (5.8 ml, 2.2 x 10-2 mmol) in anisole (0.5 ml) was added. Then EBiB (32.4 ml, 22.1´10-2 mmol) was added to initiate the polymerization. An initial sample was taken and the sealed flask was placed in thermostated oil bath at 60 oC. Samples were taken at timed intervals and analyzed by gas chromatography (GC) and gel permeation chromatography (GPC) to follow the progress of the reaction. The polymerization was stopped after 6.2 h (Mn =19,400 and Mw/Mn = 1.26, Conv. = 91%) by opening the flask and exposing the catalyst to air.
ARGET ATRP of nButyl Acrylate:
This reaction was also carried out in the presence of glucose as an exemplary organic reducing agent with the initial ratio of reagents: nBA/EBiB/Cu(II)/TPMA/glucose = 160/1/0.0078/0.03/0.1 (50 ppm Cu vs. nBA) after 44 h at 80 oC in 20 % vol/vol anisole, PnBA with Mn = 10,500 and Mw/Mn = 1.47 was formed in 48% yield (Mn,th=9,600).
ATRP of n-Butyl Acrylate from a Polyethylene Multifunctional Macroinitiator:
(Inoue, Y.; Matsugi, T.; Kashiwa, N.; Matyjaszewski, K. Macromolecules 2004, 37, 3651-3658.)
Use of a less active TREN based ligand to polymerize nBA in a grafting from a PE-macroinitiator.
[nBA]0 : [PE-MI]0 : [CuCl]0 : [CuCl2]0 : [[BA6TREN]0 = 100 : 1 : 1 : 0.05 : 1.05. Solvent: Chlorobenzene = 80 vol %. Temperature: 100 oC.
A polyethylene multifunctional macroinitiator (PE-macroinitiator), Mn = 36,000, Mw/Mn = 3.04 with 0.90 mol % of α-bromoisobutyrate functionality, was synthesized as reported in the following reference. ( Matsugi, T.; Kojoh, S.-i.; Kawahara, N.; Matsuo, S.; Kaneko, H.; Kashiwa, N. Journal of Polymer Science, Part A: Polymer Chemistry 2003, 41, 3965-3973.) ATRP was performed using standard Schlenk techniques. Solvents and monomers were degassed through bubbling with nitrogen for 30 min prior to use. A stock solution of the catalyst was prepared by dissolving CuCl (31.2 mg, 3.15 x 10-4 mol), CuCl2 (2.1 mg, 1.56 x 10-5 mol), and BA6TREN (301.8 mg, 3.30 x 10-4 mol) in chlorobenzene (5 mL). Separately, the PE-macroinitiator (119.1 mg, 3.49´10-5 mol-Br) was placed in a 25 ml Schlenk flask and then BA (0.5 mL, 3.49 x 10-3 mol), chlorobenzene (1.8 mL), anisole (0.1mL), and catalyst stock solution, (0.59 ml, 3.51 x 10-5 mol-CuCl/BA6TREN, 1.74 x 10-6 mol-CuCl2/BA6TREN) were added sequentially to the flask. The resulting mixture was warmed up to 100 oC to start the polymerization, the PE-macroinitiator dissolved in the solvent/monomer mixture within a few minutes. Polymerization was stopped after 0.5, 1.0 or 3.75 h, and the resulting graft copolymers were precipitated by addition to excess methanol. The graft copolymers were filtrated, washed with methanol and dried under vacuum at 60 oC.
Side Chain Cleavage and Analysis:
The graft copolymer (30 mg) was dissolved in chlorobenzene (1 mL) at 100 oC. n-Butanol (4 mL) and H2SO4 (3 drops) were added to the solution and the resulting mixture was stirred at 100 oC for 3 days. After cooling, OH type anionic ion exchange resin was added to neutralize the acid catalyst, and then the solution was decanted from the ion-exchange resin. After evaporation of the solvent, the residual polymer was extracted with THF, and GPC of the cleaved side chain PBA was taken using THF GPC.
ARGET ATRP of Butyl Acrylate from the Surface of a Silicon Wafer in the Presence of a Limited Amount of Air:
(Matyjaszewski, K.; Dong, H.; Jakubowski, W.; Pietrasik, J.; Kusumo, A. Langmuir 2007, 23, 4528-4531.)
A 22 mL glass vial containing initiator-modified silicon wafer and a small stir bar was charged with butyl acrylate (15.0 mL, 105 mmol) and ethyl 2-bromoisobutyrate (32.8 μL, 0.224 mmol). Then a solution of CuCl2 (0.71 mg, 0.0053 mmol) and TPMA ligand (6.5 mg, 0.013 mmol) in anisole (2 mL) was added. After sealing the vial with a rubber septum, the solution of tin(II) 2-ethylhexanoate (109 μL, 0.337 mmol) in anisole (1 mL) was injected. The initial sample was taken and the sealed vial was placed in an oil bath thermostated at 70 oC. Samples were taken at timed intervals and analyzed by GC and SEC. The polymerization was stopped after 5 h (Mn = 13,900, Mw / Mn = 1.22, conversion = 18.4%) by opening the flask and exposing the catalyst to air.
The silicon wafer was taken out for analysis of thickness, and the solution was capped and stored in dark. In order to remove the free polymer physically adsorbed onto the surface, the resulting silicon wafer was washed with methylene chloride in Soxhlet extractor for 24 hours. The thickness of the dry poly(n-butyl acrylate) brushes, measured by ellipsometry in air, was 12.3 nm. The error of the measurement was less than 0.5 nm. The grafting density (σ) was calculated using the following equation: σ = NAhρ/Mw, where Mw is the weight average molecular weight, NA is Avogadro's number, and ρ = 1.0 g/cm3 is the bulk poly(n-butyl acrylate) density.
Bulk ATRP of n-Buthyl Acrylate from 2-Bromoisobutyrate Functional Colloids:
Silica colloidal initiators (500.0 mg, 0.13 mmol), Cu(I)Br (12.0 mg, 0.130 mmol), Cu(II)Br2 (2.0 mg, 0.010 mmol), and dNbpy (121.0 mg, 0.29 mmol) were added to a 25 mL Schlenk flask containing a magnetic stir bar. The flask was fitted with a rubber septum, and the flask was evacuated (1-5 mmHg) for a period of 5 h. The flask was then backfilled with nitrogen, and the flask was evacuated again for 5 min, followed by additional backfilling with nitrogen. This evacuation/backfilling cycle was repeated, and then nBA (8.6 g, 67 mmol) (bubbled for 1 h with nitrogen before use) was added to the flask via syringe. The rubber septum fitted on the Schlenk flask was replaced with a greased glass stopper under high nitrogen purge, and the reaction mixture was homogenized by agitation on a vortex mixer for 5-10 min. The reaction flasks were then placed in a 90 °C oil bath. Samples were taken periodically via syringe for kinetic analysis of the polymerization. After 52.6 h, a monomer conversion reached 10%, as determined from gravimetric analysis. Samples were diluted with tetrahydrofuran, filtered through neutral alumina, concentrated in vacuo to a volume of approximately 10 mL, then precipitated into solution of methanol (400 mL) and deionized water (50 mL), yielding a clear viscous oil (Mn SEC cleaved pBA ) 6800; Mw/M n ) 1.26).
Miniemulsion AGET ATRP of Acrylates
CuBr2 and BPMODA were added to a round bottom flask together with the selected monomer, such as nBA or tBA. The reagents were heated under stirring to 60 oC in an oil bath in order to form the Cu(II) complex. The flask was cooled to 0 oC before the addition of an ATRP initiator, the surfactant solution and the co-surfactant. The mixture was sonicated for 1 minute and then transferred to a Schlenk flask for argon purging. After 30 minutes purging, the Schlenk flask was immersed in an oil bath thermostated at 80 oC. The polymerization was started by injection of a solution of ascorbic acid.
[ascorbic acid]:[CuII] = 0.4. [BA]:[EBiB]:[CuBr2/BPMODA] = 200:1:0.4, Temp. 80 oC; [Brij 98]:[hexadecane] = 2.3/3.6% based on monomer; based on solid content = 2%
Reation was conducted over 275 minutes and the MW remained close to theoretical up to 90% conversion and Mw/Mn = 1.2.
ATRP of n-Butyl Acrylate from Initiator-coated Silica Particles in Miniemulsion using Simultaneous Reverse and Normal Initiation (SR&NI)
0.0085g CuBr2, (3.8x10-5 mol), 0.017g BPMODA and 4.86g (5.44mL/0.0379mol) of n-butyl acrylate were added to a round bottom flask and allowed to stir at 60°C for ~20 min to dissolve the solid reagents. The solution was then cooled by immersing the flask in ice. While on ice, 20g of 5mm solution of Brij 98 in deionized water, 0.0039g purified AIBN, 0.125 mL (0.18g) hexadecane, and 0.61g of the silica particles functionalized with bromoisobutyrate were added to the flask. The mixture was sonicated for 3-4 minutes while remaining under contact with ice and then transferred to a Schlenk flask and bubbled with argon gas for 30 minutes. The flask was transferred to an oil bath heated to 80 °C and allowed to react for 6 hours. The polymerization was stopped by quickly adding the miniemulsion to methanol to precipitate the solids which were filtered for collection. Etching of silica for SEC measurements was done as reported previously.
[n-BA]:[SiO2-Br]:[AIBN]:[CuBr2]:[BPMODA] = 200:1:0.125:0.2:0.2;
In a reaction targeting higher DP, the ratio of reagents were:
[nBA]:[ SiO2-Br]:[hexadecane]:[AIBN]:[CuBr2]:[BPMODA] = 200:0.2:3.6, wt %:0.025:0.2:0.2.
Actual Mn after etching the silica cores, of the final material is in very nice agreement with theoretical at 15,900 g/mol. Mw/Mn = 1.34
Synthesis of n-Butyl Acrylate Molecular Brushes via AGET ATRP in Miniemulsion
In a typical run, the macroinitiator pBPEM (0.026 g), CuBr2 (0.0087 g) and BPMODA (0.0176 g) were dissolved in BA (5.0 g) in a round-bottom flask at 60 oC. After the formation of the Cu(II) complex, hexadecane (0.18 g) and an aqueous solution of Brij 98 (20 mL, 5 mmol/L) were added to the cooled solution before the mixture was subjected to sonication. The resulting homogenized suspension was transferred to a 25 mL Schlenk flask and purged with argon for 30 minutes. The flask was then immersed in an oil bath thermostated at 80 oC. An aqueous solution (0.5 mL) of ascorbic acid (0.0034 g) was injected into the flask to initiate the polymerization. Aliquots were taken at regular intervals to measure the conversion gravimetrically.
Miniemulsion conditions: [Brij 98]: [hexadecane] = 2.3/3.6% based on monomer; solid content = 20% (based on 100% conversion). When the ratio of ascorbic acid to Cu(II) was maintained at 0.35 and the polymerization was stopped at ~56% monomer conversion, majority of the polymers present are still single entities.
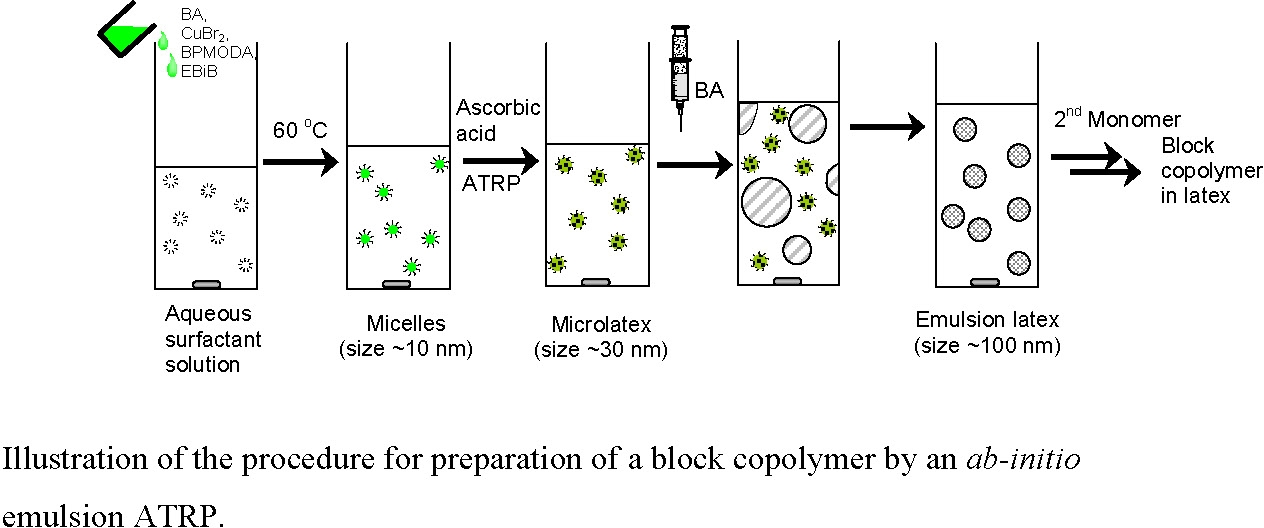
ATRP of BA in Microemulsion and Development of an ab-initio Emulsion ATRP of BA:
(Min, K.; Gao, H.; Matyjaszewski, K. Journal of the American Chemical Society 2006, 128, 10521-105.)
Step 1:
Before conducting a microemulsion polymerization, the Cu(II) complex was prepared by dissolving CuBr2 (7.8 mg, 0.035 mmol) and BPMODA (23.7 mg, 0.053 mmol) in BA (1 mL, 7 mmol) at 60 oC. The initiator, ethyl 2-bromoisobutyrate (EBiB, 10.2 μL, 0.07 mmol), was then dissolved in this complex. The resulting solution was slowly added to an aqueous solution of polyoxyethylene oleyl ether (Brij 98) (30 mL, 0.06 mol·L-1) under stirring to form an optically clear microemulsion. After purging the microemulsion with nitrogen for 30 minutes, the flask was immersed in an oil bath thermostatted at 80 oC. A pre-deoxygenated aqueous solution (0.5 mL) of ascorbic acid (2.4 mg) was injected to the microemulsion to initiate polymerization. Aliquots were withdrawn at regular intervals to measure the monomer conversion gravimetrically.
Step 2:
Development of an ab-initio Emulsion Polymerization: This microemulsion polymerization was then employed as the first step (nucleation step) in the designed "two-step" procedure to create an ab-initio emulsion ATRP. When monomer conversion reached a certain level in the microemulsion polymerization, additional monomer was added to the reaction. The polyBA-Br generated in the initial microemulsion ATRP functioned as a macroinitiator for further emulsion polymerization of additionally added monomer. Because there was no initiator or catalyst in the added monomer, monomer diffused from the droplets to the polymerizing particles.
AGET ATRP Preparation of a Block Copolymer in an ab-initio Emulsion Polymerization
An ab-initio emulsion ATRP was initiated using the same procedure as above. The second monomer, styrene, was added to the reaction when the first monomer reached ~50% conversion. An ab-initio emulsion ATRP forming a block copolymer was thereby successfully developed.
Poly(t-Butyl Acrylate)
A Typical t-Butyl Acrylate Polymerization from a Mono-functional Initiator:
tBA (Aldrich, 98%) was extracted 3 times with 5% aq. NaOH, and then washed with distilled water. After drying over CaCl2 and filtering off the drying agent, the monomer was distilled under vacuum (60 °C /60 mmHg). CuBr (39.1 mg, 2.73 x 10-4mol) and CuBr2 (3.0 mg, 1.4 x 10-5 mol) were added to a dry round-bottom flask. The flask was sealed with a rubber septum, degassed and back-filled with nitrogen three times, and left under nitrogen. Deoxygenated acetone (1 mL) was added, after which t-BA (4.0 mL, 2.7 x 10-2 mol) was added, both via syringes that had been purged with nitrogen. PMDETA (60 μL, 2.9 x 10-4 mol) was added, and the solution was stirred until the Cu complex had formed. This is easily visualized through a change of the solution from cloudy and colorless to clear and light green. After complex formation, methyl 2-bromopropionate (61 μL, 5.5 x 10-4 mol) was added to the flask, an initial sample was removed, and the flask was placed in an oil bath thermostated at 60 °C. After 320 min, a sample was dissolved in toluene, and GC analysis showed a monomer conversion of 93%.
Starting with [t-BA]:[MBrP]:[CuI][CuII]:[PMDETA] = 100:1:1:.05:1.05
The polymer had a Mn = 6,000 and Mw/Mn = 1.11.
(Note: for reactions performed at temperatures higher than 60 °C, approximately 2 % p-dimethoxybenzene or anisole, relative to the volume of monomer, was additionally added. This was to ensure conversion measurements were not affected by loss of solvent or monomer at elevated temperatures.)
Preparation of a Difunctional poly(t-butyl acrylate):
t-Butyl acrylate was purified by passing the monomer through a basic alumina column and then bubbled with N2 for 30 minutes. CuBr is charged in to the flask and after 30 min under nitrogen atmosphere, t-butyl acrylate, PMDETA and anisole are added. The solution should be light green in colour as complex formation occurs. A sample was removed to measure the initial monomer/internal standard ratio used afterward to determine the conversion. Dimethyl 2,6-dibromoheptanedioate (DMDBHD)was then added as initiator and the flask was placed in an oil bath thermostated at 60°C. After the desired conversion was attained the flask was then removed from the oil bath, the solution was diluted in tetrahydrofuran and purified by passing through a neutral alumina column. The solvent and monomer were then removed under vacuum at 45 °C.
a) Mole Ratio: [tBA]:[DMDBHD]:[CuBr]:[PMDETA] = 100:1:0.25:0.25;
25% acetone; held at 60°C for 370 minutes.
Conv. 53%; Mn = 6,800, Mw/Mn = 1.16
b) Mole Ratio: [tBA]:[DMDBHD]:[CuBr]:[PMDETA] = 300:1:0.33:0.33
Conv. = 37.2%; Mn =15,000, Mw/Mn = 1.25
CuBr (12 mg, 8.5 x 10-5 mol) and DMDBHD (55 mg, 1.7 x 10-4 mol) were added to a 10 ml round bottom flask. The flask was degassed and back-filled with nitrogen three times before introducing deoxygenated tBA (5.0 ml, 3.4 x 10-2 mol) and anisole (100 ml, as an internal standard) via purged syringes. PMDETA (18 ml, 8.5 x 10-5 mol) was added and the copper complex formed. The solution was heterogeneous. An initial sample was removed and the flask was placed in an oil bath thermostated at 60 ºC. After 6.5 hours, a sample was removed, dissolved in toluene and GC analysis was performed.
Monomer conversion was 79%, with a Mn = 25,100; Mw/Mn = 1.24.
AGET ATRP of t-Butyl Acrylate in a Miniemulsion:
CuBr2 and BPMODA were charged to a round bottom flask together with the selected monomer, such as nBA or tBA. The reagents were heated to 60oC in an oil bath in order to form the Cu(II) complex. The flask was cooled to 0oC before the addition of an ATRP initiator, the surfactant solution and the co-surfactant. The mixture was sonicated for 1 minute and then transferred to a Schlenk flask for argon purging. After 30 minutes purging, the Schlenk flask was immersed in an oil bath thermostated at 80oC. The polymerization was started by injection of a solution of ascorbic acid. The ascorbic acid was added slowly over a 10 minute period. This resulted in the reaction attaining more linear kinetics than that obtained when all of the ascorbic acid was added at the very beginning of the reaction. In order to leave some excess of Cu(II) species to regulate ATRP, the sub-stoichiometric amount of the reducing agent was used, a ratio of Cu/ascorbic acid = 1/0.4 worked quite well.
Lauryl Acrylate
(Beers, K. L.; Matyjaszewski, K. J. Macromol. Sci., Pure Appl. Chem. 2001, A38, 731-739.)
Use of a ligand that increases the solubility of the catalyst complex in the monomer is suggested, e.g. an alkyl substituted bipyridine ligands instead of PMDETA yields a homogeneous catalyst solution without requiring a co-solvent. A low rate of termination in Lauryl Acrylate (LA) polymerization leads to high molecular weight polymer at low conversions if additional deactivating species, Cu(II) a minimum of mole 4%, is not present in the solution when the reaction is initiated.
[LA] = 3.5 M in toluene, [MBrP] = 17.3 mM, [CuBr(dNbpy)2] = 17.3 mM, [CuBr2] = 0.7 mM, T = 90°C. Conversion 89% after 500 min. Mw/Mn = 1.2.
ATRP of Lauryl Acrylate
LA (2.5 mL; 9.2 mmol) and dNbpy (0.0755g; 0.18 mmol) were dissolved in 2.5 mL toluene and nitrogen gas was bubbled through the solution while stirring for 45 minutes. CuBr (0.0129 g; 0.09 mmol) was added and an initial sample was taken by syringe. The solution was bubbled with nitrogen for an additional 10 minutes until homogeneous and the flask was placed in a 90°C oil bath. Methyl 2-bromopropionate (MBrP; 10 mL) was added and samples were removed at timed intervals. After 6.75 hours, the conversion was 59 % (1H NMR). Mn,th = 14,200; Mn,ex = 12,400; Mw/Mn = 1.26.
2-Hydroxyethyl Acrylate (HEA):
(Coca, S.; Jasieczek, C. B.; Beers, K. L.; Matyjaszewski, K. J. Polym. Sci., Part A: Polym. Chem. 1998, 36, 1417-1424.)
2-Hydroxyethyl acrylate (HEA) was purified by first dissolving the monomer in water (25% by volume). Hydroquinone (0.1%) was then added to the solution to inhibit thermal polymerzation. The solution was extracted with hexane (10 times) to remove diacrylate, and the aqueous solution was salted (250 g/L NaCl). The monomer was then separated from the aqueous phase by ether extraction (4 times) to remove acrylic acid. Hydroquinone was added to the ether solution. CaSO4 drying agent was used to remove traces of water before evaporation of the ether phase. The purified monomer was subsequently kept over molecular sieves and distilled under pressure immediately prior to use. Methyl 2-bromopropionate (MBrP), di-ethyl 2-methyl-2-bromomalonate (DEMBM), CuBr (98%, and 2,2'-bipyridine ‘‘CuX/2L'' complex. Halogenated initiators and (bpy) were used as received from Aldrich.
polymerizations were conducted
Bulk Polymerizations:
These polymerizations were conducted in sealed glass tubes. Typical ratios of the reactants were M : I : Cu : L = 100 : 1 : 1 : 2. The initiators used were either MBrP or diethyl 2-methyl-2-bromomalonate (DEMBM). All reagents were added to the glass tube, and the mixture was degassed three times before sealing tubes under vacuum. Reactions were conducted in an oil bath regulated at 900C. Tubes were removed at regular intervals for kinetic experiments, the resulting polymer was dissolved in DMF, and samples were injected directly into the SEC. The solution was additionally passed over alumina to remove the catalyst and dried in a vacuum oven overnight to remove monomer and DMF to isolate the polymer for NMR (DMSO-d6) and MS analysis.
Reaction Conditions:
0.0337 g (0.2 mmol) of bpy, 0.0154 g (0.1 mmol) of CuBr, 12 mL (0.1 mmol) of MBrP and 0.5 mL (4.3 mmol) of HEA were combined in a reaction tube and degassed via three freeze-pump-thaw cycles. The tube was then sealed under vacuum and placed in an oil bath 90 oC for 2 h. The tube was then immediately frozen in liquid nitrogen and broken open. Part of the contents was dissolved in DMSO-d6, and the rest, in DMF. The deuterated sample was used to determine conversion by 1H-NMR. About 0.5 mL of the DMF solution was filtered through a 0.2 mm filter with one drop of diphenyl ether as standard and injected directly into the GPC. The remaining DMF solution was passed over alumina and dried in a vacuum oven at 600C overnight. Part of the remaining sample was dissolved in DMSO-d6 to determine molecular weight from NMR by end group analysis and the residual concentration of DMF, and the rest was used for preparation of MALDI samples. Mn = 3,800 (NMR), Mw/Mn 1.15; conversion 91%.
Polymerizations Using Water as a Solvent:
These polymerizations were conducted in a 1:1 (v/v) ratio of HEA/H2O. The ratio of reactants was M : I : Cu : L = 100 : 1 : 1 : 3.
The reaction mixture was degassed twice to remove traces of oxygen before tubes were sealed. Polymerization was carried out at 90 oC, and the reaction was terminated after 12 h. The tube contents were dried over MgSO4 and dissolved in DMF. The Cu/bpy complex was homogeneous in both the bulk and aqueous polymerizations forming a brown solution that is characteristic of Cu(I) complexes.
Monomer conversion was 79%, with a Mn = 25,100; Mw/Mn = 1.24.
HEA-TMS (2-(trimethylsilyloxy)ethyl acrylate):
(Muehlebach, A.; Gaynor, S. G.; Matyjaszewski, K. Macromolecules 1998, 31, 6046-6052.)
2-Trimethylsilyloxyethyl acrylate was synthesized by silylation of 2-hydroxyethyl acrylate (50 mL, 435.3 mmol) with trimethylsilyl chloride (61 mL, 479 mmol) in CH2Cl2/NEt3 (500 mL/73 mL) at 0 °C under Ar and then allowed to come to room temperature. The solution was filtered to remove NEt3·HCl and the CH2Cl2 was removed by distillation. The product was filtered again, dissolved in EtOAc (300 mL), which was washed 3 times with water, the EtOAc solution was dried and EtOAc evaporated off, and the product was distilled in vacuo (103 °C/38 mmHg). The difunctional initiator bis(2-bromopropionyloxy)ethane (F ) 1.66 g/cm3) was prepared by esterification of ethylene glycol with 2-bromopropionyl bromide and NEt3.
Polymerization Procedures: The polymerizations were conducted in predried round-bottom flasks. CuBr was added and the flask tightly closed with a rubber septum. After the air was removed by evacuation and purging with Ar (2 cycles), the monomer(s), solvent(s), internal standard(s) (for the conversion measurements by GC), and/or the ligand were added via syringe. The mixture was stirred and purged with Ar for 10 min and then, via syringe, the initiator was added and the flask placed in a preheated oil bath. After specified time intervals, samples (ca. 10 mg) were taken to determine conversions via 1H NMR. After the target conversion was reached, the flask was removed from the oil bath and the reaction mixture diluted (1:1 to 1:4) with THF or DMF. The solution was filtered through a column of neutral alumina (Fisher-Scientific, 80-200 mesh) to remove the catalyst and a sample taken for SEC analysis. The rest of the solution was concentrated by rotary evaporation, and the polymer was dried in vacuo at 60-80 °C (p < 0.1 mmHg).
Homopolymerization of HEA-TMS:
[M]:[I]:[Cu] = 50:1:0.5
38.0 mg (0.266 mmol) of CuIBr was weighed into a 10 mL round-bottom flask, which was sealed with a rubber septum, and the air was removed by evacuation and purging with Ar (2 cycles). 5.26 mL (5.0 g, 26.6 mmol) of the monomer TMS-HEA was added via syringe, followed by 55.4 μL (46 mg, 0.265 mmol) of the ligand, PMDETA. The mixture was stirred and purged again with Ar for 10 min, and then, via syringe, 59 μL (88.5 mg, 0.53 mmol) of the initiator MBrP was added and the flask placed in the preheated oil bath at 80 °C. After 20 min the conversion (determined by 1H NMR) reached >95% and the flask was removed from the oil bath. The reaction mixture was diluted 1:2 with THF and the solution filtered through an Al2O3 column. A sample was taken for SEC analysis, while the rest of the solution was concentrated in the rotary evaporator and the polymer dried in vacuo at 60 °C overnight (p < 0.1 mmHg).
Yield: 3.40 g (68%). SEC: Mn = 9,390; Mn = 9,415; Mw/Mn = 1.20. DSC: Tg = -43 °C.
Hydrolysis of Poly(HEA-TMS):
The homopolymers of HEA-TMS were hydrolyzed using a catalytic amount of HCl in THF/H2O. The degree of hydrolysis was >99% as determined by 1H NMR. The byproduct, hexamethyldisiloxane, accumulated at the top of the reaction mixture and was easily separated. The polymers were water soluble and contained a small amount of NaCl (<5%) due to neutralization of HCl with NaOH.
Homopolymerization of HEA-TMS at 80 °C in Bulk:
(I:Cu:L = 1:0.5:0.5)
Sample [M]:[I] time (h) Mn,th Mn,NMR Mn,ex Mw/Mn
AM2-A 25:1 0.3 4700 4700 4810 1.24
AM2-B 50:1 0.3 9400 9600 9390 1.20
AM2-C 100:1 0.7 18900 >12000 17030 1.26
AM2-A 200:1 2.75 37700 >12000 33000 1.24
High Molecular Weight P(HEA-TMS)
Stoichiometry of HEMA-TMS : EBiB : CuCl : CuCl2 : dNbpy = 1400 : 1 : 2 : 0.2 : 4.4, 10 vol % anisole, T = 90 °C.
There was only a slight coupling shoulder observed while high molecular weights were obtained.
Mn = 141,000; (DPn = 697); Mw/Mn = 1.11
Glycidyl Acrylate
(Matyjaszewski, K.; Coca, S.; Jasieczek, C. B. Macromol. Chem. Phys. 1997, 198, 4011-4017.)
Glycidyl acrylate was purified by vacuum distillation over iBu3Al. The purified monomer was stored over mole sieves. dNbpy was used the ligand (see below for synthesis) and the initiator was MBrP. Typical ratio of reagents were M : I : Cu : L = 100 : 1 : 0.03 : 0.06. Bulk polymerizations were conducted in sealed glass tubes. The reagents were added to the tubes and the mixture was degassed three times before the tubes were sealed under vacuum. The reactions were conducted at 90 °C. Tubes were removed periodically to follow kinetics and it was possible to prepare high MW polymer with low PDI.
[M] Conv. Mn,th Mn,exp Mw / Mn
25 95% 3,440 4,320 1.23
150 98% 25,000 27,500 1.21
300 98% 50,000 52,800 1.20
