Simultaneous Reverse & Normal ATRP (SR&NI)
SR&NI, was developed to overcome the problems "reverse" ATRP has with:
- incorporation of an α-functionality into the chain end of linear copolymers,
- preparing polymers with targeted MW,
- preparation of polymeric materials with more complex architecture and,
- preparation of composite or hybrid materials.
These problems with "reverse" ATRP were amplified by a desire to use even more active catalyst systems in an ATRP reaction, since more active catalyst complexes are more easily oxidized by exposure to air.
In SR&NI a low concentration of an active catalyst complex is generated by decomposition of a standard free radical initiator, such as AIBN in a standard reverse ATRP procedure, while the majority of the polymer chains are initiated from an added alkyl (pseudo)halide via a normal ATRP process. This allows very active catalysts to be added to the reaction in their stable form and the bulk of the polymer to be formed from the added alkyl halide initiator.
The following schematic is a summary of the normal ATRP and reverse ATRP initiation mechanisms, shown above, illustrating how both initiation procedures are employed in SR&NI. The reagents shown in red are the reagents that are added to a SR&NI reaction.
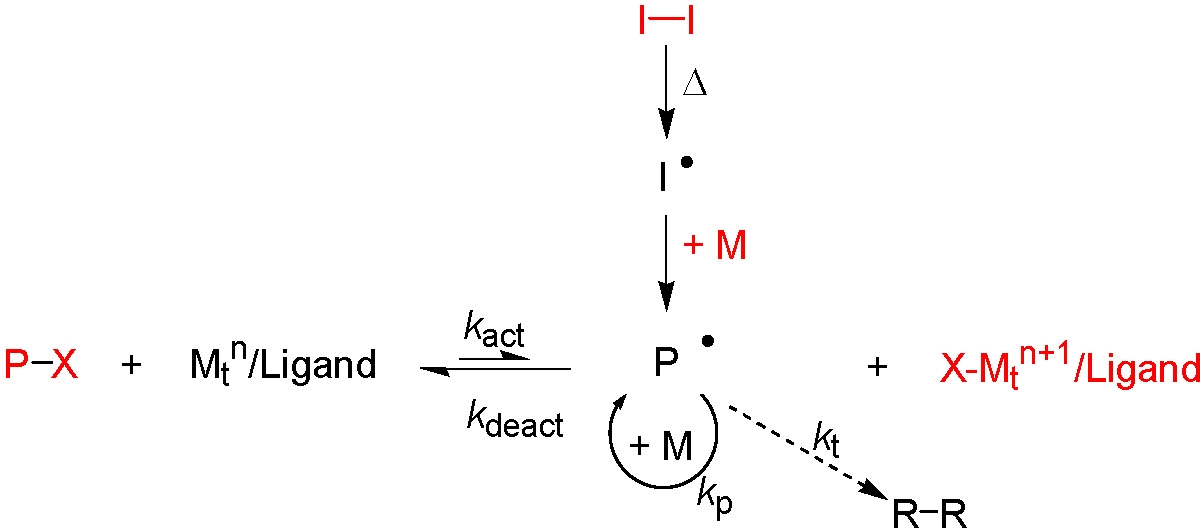
The first formed radicals drive the reverse ATRP initiation reaction where an active catalyst complex in the higher oxidation state is reduced to the activator state by reaction with the formed radicals (kdeact) but the bulk of the polymer chains are initiated by the normal ATRP initiation mechanism from the added standard initiator (P-X) providing polymers with a high mole fraction of functional α-terminal functionality.
The degree of polymerization is predominately controlled by the concentration of initially added alkyl halide, as expressed in the following equation, where f is the initiation efficiency of the added free radical initiator.
![]()
SR&NI was initially developed for bulk polymerization using macroinitiators to prepare block copolymers.(1) However SR&NI was quickly adapted to miniemulsion systems where the ability to add an oxidatively stable catalyst precursor to the precursor of the emulsion prior to sonication simplifies the laboratory procedure(2-6) and allows the preparation of block, star, graft and hybrid copolymers in heterogeneous media.
The kinetics of the various ATRP systems, including normal ATRP, normal ATRP in the presence of initially-added CuII, reverse ATRP and SR&NI ATRP were modeled using Predici software. The instantaneous kinetic chain length was introduced for ATRP and was used for the prediction of control over the polymerization. Equations were derived to establish the radical concentration at the quasi-steady-state. Normal ATRP experiences a continuous decrease of radical concentration leading to a decrease in the rate or polymerization; in contrast, SR&NI ATRP undergoes a continuous increase in radical concentration leading to an increase of the polymerization rate. All of these ATRP methods can afford a relatively fast polymerization rate while retaining good control over the polymerization.(7)
Indeed, based on the simple commercially viable, scalable, SR&NI ATRP procedure process improvements continued, even after the lower ppm catalyst systems discussed below were developed. One development was photoinduced SR&NI ATRP. It was observed that the type and concentration of photoinitiators directly influences the photoinduced reverse ATRP. Photoinduced SR&NI ATRP proceeds in a well-controlled manner under UV light at room temperature as was evident from polymerization kinetics and chain extension studies. Indeed better agreement between the experimental and theoretical MW with a narrow dispersity was observed with the photoinduced SR&NI ATRP system.(8)
Composite materials were prepared by in situ SR&NI ATRP in miniemulsion due to its abundant advantages for encapsulation of inorganic materials. Successful SR&NI ATRP was carried out using 4,4'-dinonyl-2,2'-bipyridine (dNbpy) as a hydrophobic ligand and cetyltrimethylammonium bromide (CTAB) as an effective cationic surfactant at high temps. A homogeneous distribution of droplets and particles with sizes in the range of around 170 nm was determined by dynamic light scattering (DLS) analysis, and confirmed by SEM.(9) Another successful procedure for preparation of hybrid particles using SR&NI ATRP was demonstrated by the introduction of a double bond containing modifier on the surface of silica nanoparticles followed by grafting through polymerization of styrene.(10) The authors utilized hexamethyldisilazane (HMDS) for surface modification of hydrophilic silica aerogel nanoparticles then, the resultant modified nanoparticles were used for in situ polymerization of styrene by SR&NI ATRP to synthesize tailor-made polystyrene nanocomposites.(11) They later employed 3-(trimethoxysilyl)propyl methacrylate (MPS) to functionalize the surface of silica particles for the successful grafting through copolymerization of styrene and butyl acrylate.(12) The utility of the procedure was recently further exemplified by the preparation of a denitrification adsorbent with large surface area and specific pore structure for the removal of indole from fuel oil by the synthesis of molecularly imprinted polymers (MIPs) by surface imprinting methods.(13)
REFERENCES
(1) Gromada, J.; Matyjaszewski, K. Macromolecules 2001, 34, 7664-7671.
(2) Matyjaszewski, K.; Gromada, J.; Li, M. In PCT Int. Appl.; (Carnegie Mellon University, USA). WO 03/031481, 2003; p 46 pp.
(3) Li, M.; Matyjaszewski, K. Journal of Polymer Science, Part A: Polymer Chemistry 2003, 41, 3606-3614.
(4) Li, M.; Min, K.; Matyjaszewski, K. Macromolecules 2004, 37, 2106-2112.
(5) Li, M.; Jahed, N. M.; Min, K.; Matyjaszewski, K. Macromolecules 2004, 37, 2434-2441.
(6) Min, K. E.; Li, M.; Matyjaszewski, K. Journal of Polymer Science, Part A: Polymer Chemistry 2005, 43, 3616-3622.
(7) Tang, W.; Matyjaszewski, K. Macromolecular Theory and Simulations 2008, 17, 359-375.
(8) Tasdelen, M. A.; Uygun, M.; Yagci, Y. Macromolecular Chemistry and Physics 2011, 212, 2036–2042.
(9) Khezri, K.; Haddadi-Asl, V.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Journal of Polymer Research 2012, 19, 1-10.
(10) Khezri, K.; Haddadi-Asl, V.; Roghani-Mamaqani, H. Nano 2014, 9, 1450023.
(11) Khezri, K.; Mahdavi, H. Microporous and Mesoporous Materials 2016, 228, 132-140.
(12) Sarsabili, M.; Kalantari, K.; Khezri, K. Journal of Thermal Analysis and Calorimetry 2016, 126c, 1261-1272.
(13) Yang, W.; Cao, Y.; Ni, X.; Hunag, W.; Xu, W. Adsorption Science & Technology 2015, 33, 441-456.
