Solvent Effects and Selection of a Catalyst for Aqueous Media
The performance of copper-based ATRP catalysts can be predicted based on the stability constants of the CuII and CuI complexes with the chosen ligand L (βII and βI, respectively). Both βII and βI should be large in order to prevent catalyst deactivation through competitive coordination of monomer and/or polymer that are present in such large molar excess. A high βII/βI ratio is required for high catalytic activity.
More halidophilic CuII-L complexes provide more active catalyst complexes and better control over the polymerization due to the fact that there is a sufficient concentration of deactivator present in the system.
If the ATRP is to be carried in aqueous media, in addition to the above requirements, the ratio βII/(βI)2[L] should be low in order to prevent disproportionation of the CuI complex. Acidic monomers may be polymerized, if the ligands meet all outlined requirements and are as weakly basic as possible.
CuI complexes are generally unstable in aqueous media and tend to disproportionate. For instance, the disproportionation of non-complexed CuI in water is characterized by an equilibrium constant as large as Kdisp = 106.(1) Addition of excess ligand L, or a ligand substitute, can significantly affect the equilibrium and the equilibrium constant changes to a new value, determined by the stabilization of CuII relative to CuI upon coordination with L.
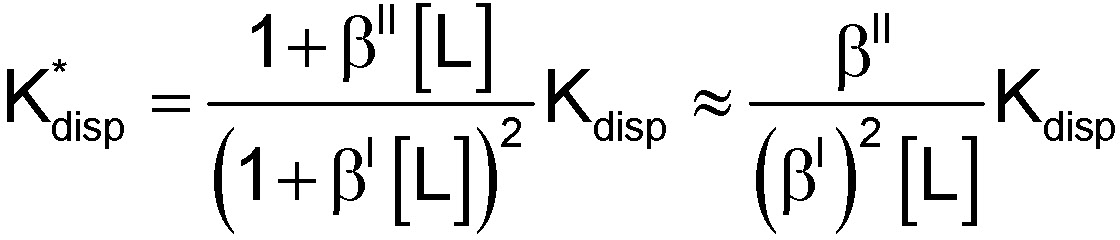
Therefore, for ligands forming 1:1 complexes with copper ions, the activity of the catalyst is proportional to βII/βI whereas the tendency of the CuI complex to disproportionate in aqueous solution (which should be minimized) depends on the ratio βII/(βI)2[L].(2) Thus, a "map" can be constructed that can be used to select a ligand for aqueous ATRP that forms an active complex yet remains stable towards disproportionation.
More details on the Cu(I) disproportionation reaction, including the effect of both ligand and solvent on it are provided in a review paper, describing various redox process related to ATRP.(3)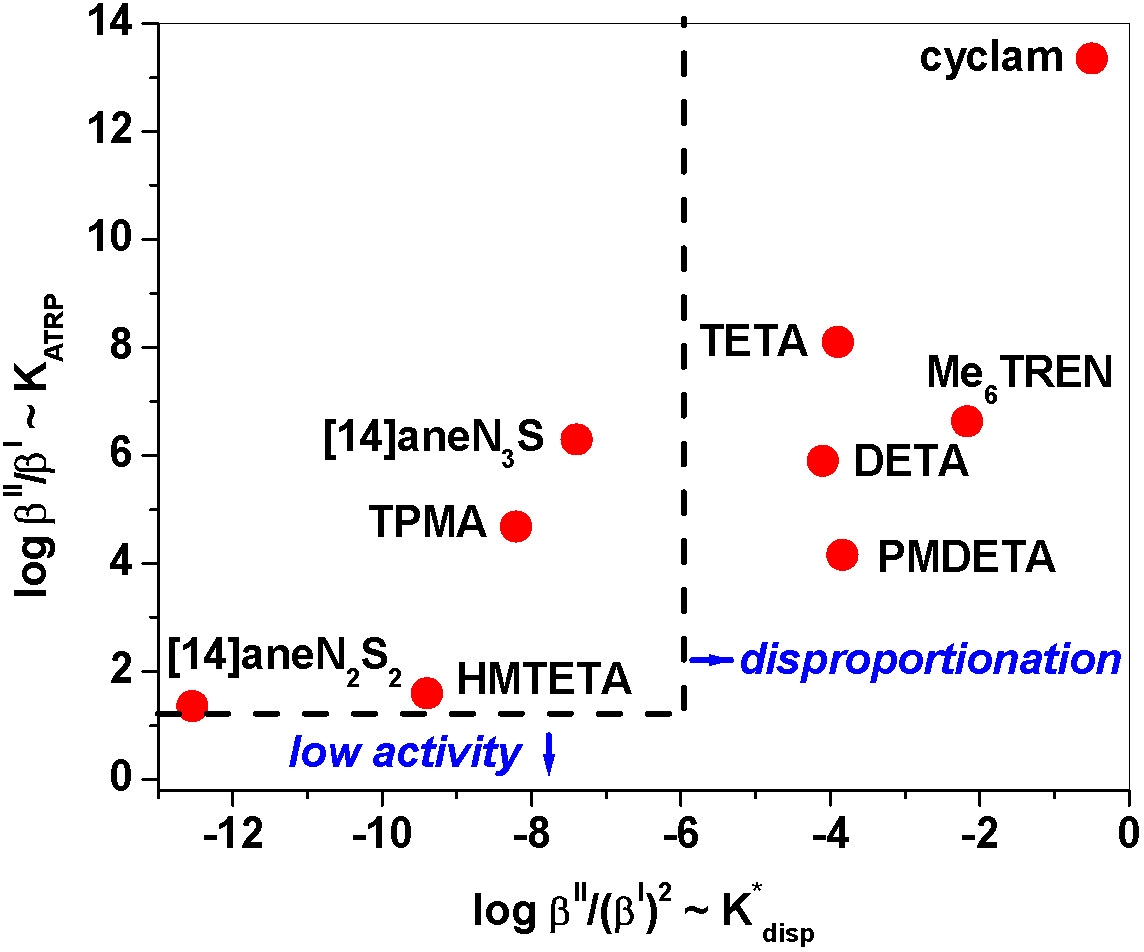
Correlation between ATRP catalytic activity and tendency for disproportionation for several CuI complexes.(4)
The figure shows that while the CuI/PMDETA complex is very active it is not suitable for aqueous ATRP due to very fast disproportionation. On the other hand, ligands such as bpy, HMTETA, and TPMA can be used in aqueous media, although with rather different activities. If necessary, catalyst disproportionation in water can be suppressed by using an appropriate co-solvent or by addition of a pseudo ligand that will stabilize CuI vs. CuII, such as pyridine, which allows aqueous ATRP of ionic monomers such as sodium 4-styrenesulfonate and 2-(N,N,N-trialkylammonio)ethyl methacrylate salts, to be conducted.(3-4)
Other Catalyst Systems
Many different catalyst systems have been used in these studies. The most frequently used metals are Cu, Fe, and Ru, although other transition metals that can undergo a one electron redox transition have also been examined.
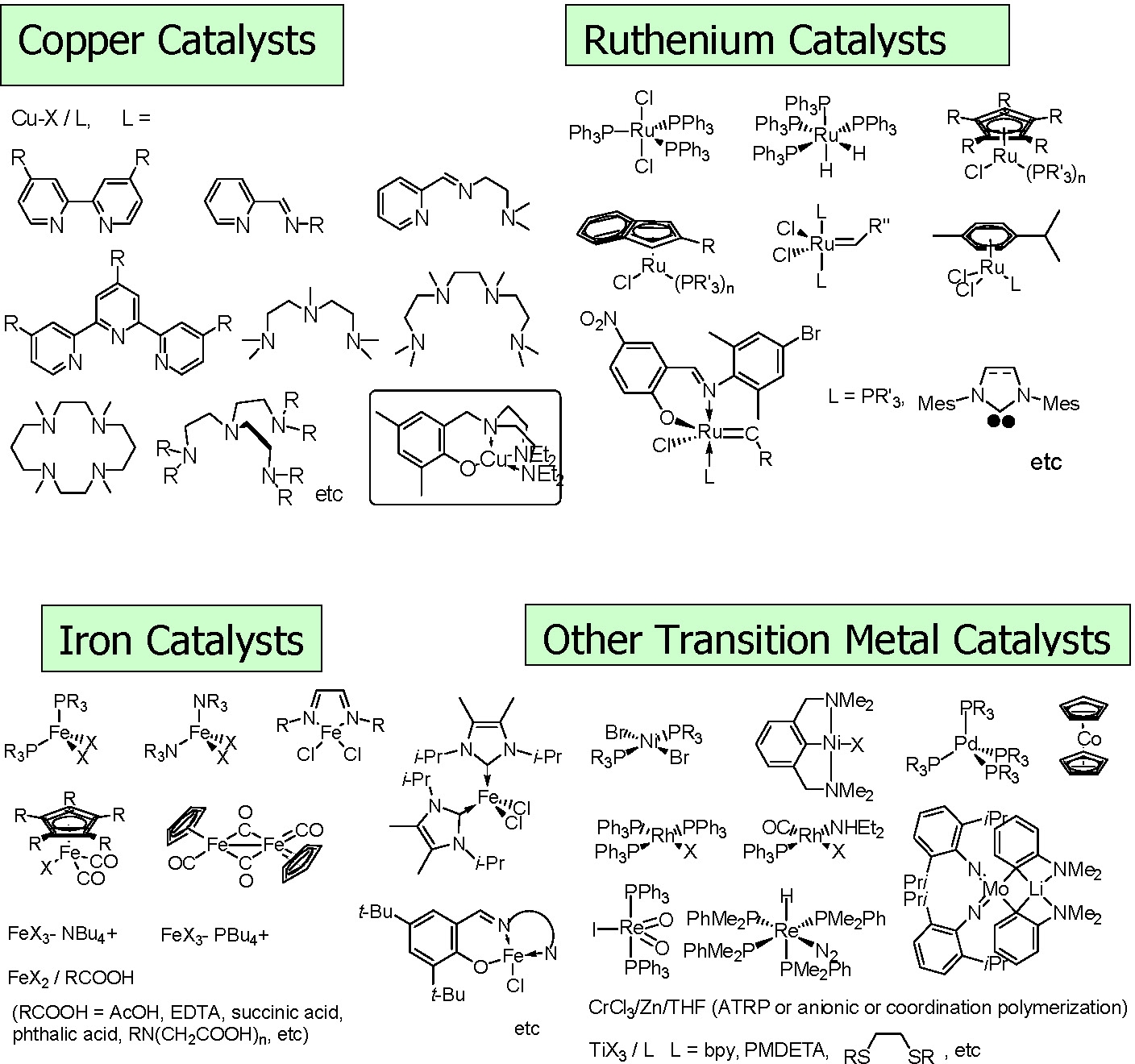
Examples of an iron based system with comparable activity to the copper based catalysts developed in the Matyjaszewski laboratories were developed by Gibson.(5-6)
The target for the continued work on the development of more active catalysts for ATRP has been the preparation of a higher quality product by reducing the catalyst loading and running under more economical conditions by lowering the reaction temperature and continually seeking to increase the range of monomers that can be controllably copolymerized.
Some Side Reactions
A number of side reactions have been examined using the kinetic tools described above. These include the association/dissociation equilibrium between the vinyl monomers and the catalyst complex.(7) The enthalpy and entropy of coordination and the relative binding constants of methyl acrylate (MA), 1-octene (Oct), styrene (Sty), and methyl methacrylate (MMA) with Cu/PMDETA were determined.(8) The affinity of the monomers to the CuI center decreased in the order MA > Oct > Sty > MMA and the consequence of monomer coordination to CuI in atom transfer radical polymerization (ATRP).
The reactivity of the selected comonomers in an ATRP, and in conventional free radical copolymerization reactions are not significantly affected by monomer coordination to the transition metal complex. However, these reactions become more important as the concentration of the catalyst complex in the reaction medium is reduced to ppm levels as in ARGET and ICAR ATRP.(9) Under such dilute conditions, catalysts with tridentate ligands such as PMDETA (to which monomers can coordinate) do not perform as well as catalysts with tetradentate ligands of otherwise comparable activity.
Other concurrent reactions that may occur during ATRP, and could affect catalyst efficiency, include disproportionation of the CuI catalyst in aqueous or polar media, transfer reactions associated with the complexing ligand,(3,10) and solvent coordination.(11,12)
Indeed with the recent development of new initiation techniques in ATRP that allow catalysts to be employed at unprecedented low concentrations (approximately10 ppm), a thorough understanding of competitive equilibria that can affect catalyst performance is becoming increasingly important and includes:
i) factors affecting the position of the ATRP equilibrium;
ii) dissociation of the ATRP catalyst at high dilution and loss of deactivator due to halide dissociation;
iii) conditional stability constants as related to competitive monomer, solvent, and reducing agent complexation as well as ligand selection with respect to protonation in acidic media; and
iv) competitive equilibria involving electron transfer reactions, including the radical oxidation to carbocations or reduction to carbanions, radical coordination to the metal catalyst, and disproportionation of the Cu|-based ATRP activator.
As noted above, one concern is the possibility of Outer Sphere Electron Transfer, or the reduction / oxidation of radicals by the catalysts to carbanions / carbocations (see below).(13) As noted in reference 13, the continuous development of more active and stable catalysts in ATRP has required an increasingly thorough knowledge of concurrent electron transfer reactions that can affect catalyst performance. One must select the appropriate catalyst for the targeted monomers; e.g. CuI/Me6TREN is too "reducing" for acrylonitrile and CuI/bpy is too "oxidizing" for vinyl ether. These side reactions can be viewed as arising from reactions due to formation of carbocations or carbanions and radical coordination to the metal catalyst resulting in the interplay of controlled radical polymerization mechanisms.
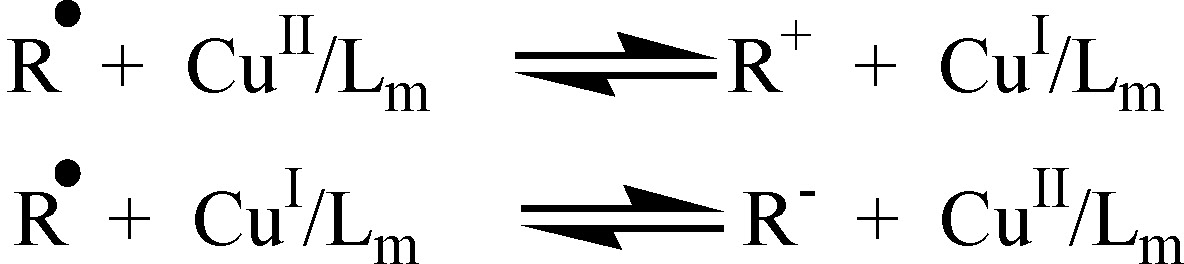
Outer Sphere Electron Transfer as a Side Reaction in ATRP
Initial attempts to directly polymerize acidic monomers (such as acrylic or methacrylic acid) by ATRP failed due to interactions of the acids with the catalyst leading to complete loss of activity. The concept of conditional stability constants(14,15) allows the calculation of the stability of the ATRP catalyst in acidic solutions. Generally, complexes formed with very basic ligands are rather sensitive to the pH of the medium, although in the case when the ligand forms particularly stable copper complexes, protonation of the catalyst complex can become negligible. A graphical representation of the dependence of the conditional stability constant β1*(CuIIL) of a catalyst complex as a function of acid concentration is shown below.
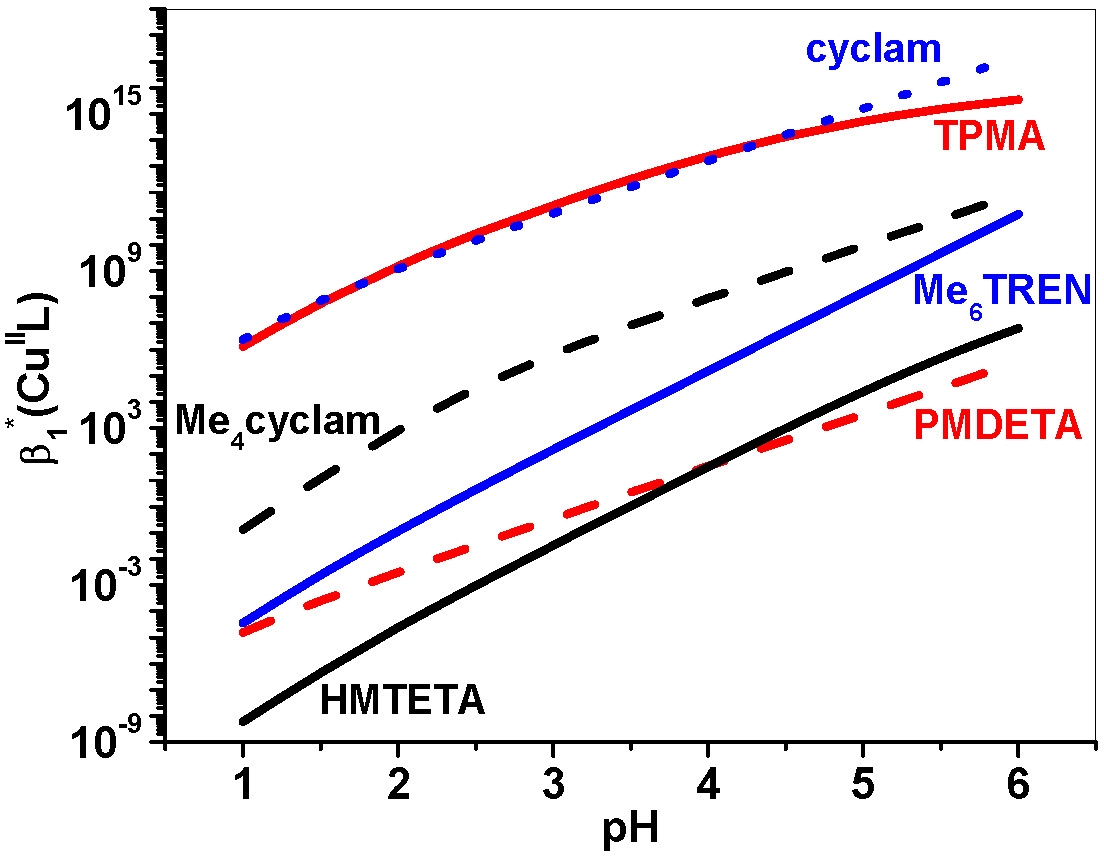
TPMA is a good starting point for copolymerization of acidic monomers.
Recently, as discussed elsewhere on the web page [Electrochemical Control over an ATRP, (eATRP)], a well-controlled eATRP was carried out in water with relatively low catalyst concentrations.(16) A low CuI to CuII ratio was maintained by slowly reforming CuI species using a cathodic current. However, other methods can be used to slowly and continuously reduce CuII species to regain the CuI activators. One of the simplest methods is to continuously regenerate the CuI species in the presence of thermal radical initiators, as in ICAR ATRP.
ICAR ATRP in water
Low catalyst concentrations and benign solvents are desirable to reduce the environmental impact of ATRP(17) and for the first time, Cu catalyst concentrations of 100 ppm and lower were used in aqueous media to prepare well-defined macromolecules.(18) The effect of various reaction parameters including as the concentration of a halide salt, selected catalyst and target degree of polymerization on homopolymerization of (EO)XOMEA were investigated. One challenge associated with copper based ATRP in water is the dissociation of deactivator complexes to give bromide ions and Cu(II)/L complexes that cannot deactivate the growing radicals.(12,19) Adding a large excess of a salt with a halide anion (e.g. tetraethylammonium bromide, TEABr) shifts the equilibrium back towards the deactivator complex. The results should be independent of the cation used. This implies that cations such as sodium or potassium could be used, or even polymerizable cations such as those from quarentized 2-(dimethylamino)ethyl methacrylate. The copper halide catalyst with tris(pyridin-2-ylmethyl)amine (TPMA) ligand gave stable CuI complexes in water, without any significant disproportionation,(3,20-21) and polymers of oligo(ethylene oxide) methyl ether acrylate were synthesized with low dispersity (Mw/Mn=1.15–1.28) using 20-100 ppm of an active CuBr/TPMA based catalyst in the presence of excess bromide anions. All polymerizations were performed at 44 °C, which corresponds to a 10 h half-life for the 2,2'-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride (VA-044) azo-initiator, higher temperatures are expected to be too high for the majority of polymer protein conjugate applications temperatures are expected to be too high for the majority of polymer protein conjugate applications(22) which are a target for controlled grafting from polymerizations. Cu catalyst concentrations from 20 to 100 ppm chains grow uniformly. When 5 ppm catalyst was used, the molecular weights were higher than in the other polymerizations, indicating a loss of control at approximately 5 ppm due to a lower concentration of deactivator, and longer transient radical lifetimes.(23-24) ICAR ATRP was also used to graft an A(EO)XOMe, where x is ca. 8.4, polymer directly from a bovine serum albumin (BSA) protein, to prepare a protein-polymer hybrid (PPH).(25-26) In this case, a BSA protein with ca. 30 cleavable initiating sites was used as a macroinitiator.(22) The polymer molecular weights, determined, after cleavage from protein, resulting in a final values of Mn ≈ 55,000 and Mw/Mn = 1.15 Although ATRP methods were recently optimized for the synthesis of well-defined polymers from proteins(22) the method presented here uses ca. 500 times lower catalyst concentrations, facilitating purification of the PPH.
REFERENCES
(1) Datta, D. Indian J. Chem., Sect. A 1987, 26A, 605-606.
(2) Tsarevsky, N. V.; Matyjaszewski, K. ACS Symposium Series 2006, 937, 79-94.
(3) Tsarevsky, N. V.; Braunecker, W. A.; Matyjaszewski, K. Journal of Organometallic Chemistry 2007, 692, 3212-3222.
(4) Tsarevsky, N. V.; Matyjaszewski, K. Chemical Reviews 2007, 107, 2270-2299.
(5) O'Reilly, R. K.; Gibson, V. C.; White, A. J. P.; Williams, D. J. Journal of the American Chemical Society 2003, 125, 8450-8451.
(6) O'Reilly, R. K.; Shaver, M. P.; Gibson, V. C.; White, A. J. P. Macromolecules 2007, 40, 7441-7452.
(7) Braunecker, W. A.; Tsarevsky, N. V.; Pintauer, T.; Gil, R. R.; Matyjaszewski, K. Macromolecules 2005, 38, 4081-4088.
(8) Gromada, J.; Spanswick, J.; Matyjaszewski, K. Macromolecular Chemistry and Physics 2004, 205, 551-566.
(9) Matyjaszewski, K.; Jakubowski, W.; Min, K.; Tang, W.; Huang, J.; Braunecker, W. A.; Tsarevsky, N. V. Proceedings of the National Academy of Sciences of the United States of America 2006, 103, 15309-15314.
(10) Braunecker, W. A.; Pintauer, T.; Tsarevsky, N. V.; Kickelbick, G.; Matyjaszewski, K. J. Organomet. Chem. 2005, 690, 916-924.
(11) Huang, J.; Pintauer, T.; Matyjaszewski, K. Journal of Polymer Science, Part A: Polymer Chemistry 2004, 42, 3285-3292.
(12) Tsarevsky, N. V.; Pintauer, T.; Matyjaszewski, K. Macromolecules 2004, 37, 9768-9778.
(13) Matyaszewski, K. Macromol. Symp. 1998, 134, 105-118.
(14) Schwarzenbach, G. Die komplexometrische Titration. 2nd ed, 1956.
(15) Ringbom, A. Chemical Analysis. Vol. XVI. Complexation in Analytical Chemistry, 1963.
(16) Magenau, A. J. D.; Strandwitz, N. C.; Gennaro, A.; Matyjaszewski, K. Science 2011, 332, 81-84.
(17) Tsarevsky, N. V.; Matyjaszewski, K. Chemical Reviews 2007, 107, 2270-2299.
(18) Konkolewicz, D.; Magenau, A. J. D.; Averick, S. E.; Simakova, A.; He, H.; Matyjaszewski, K. Macromolecules 2012, 45, 4461-4468.
(19) Tsarevsky, N. V.; Braunecker, W. A.; Vacca, A.; Gans, P.; Matyjaszewski, K. Macromolecular Symposia 2007, 248, 60-70.
(20) Bortolamei, N.; Isse, A. A.; Magenau, A. J. D.; Gennaro, A.; Matyjaszewski, K. Angew. Chem., Int. Ed. 2011, 50, 11391-11394, S11391/11391-S11391/11315.
(21) Zhang, Y.; Wang, Y.; Peng, C.-h.; Zhong, M.; Zhu, W.; Konkolewicz, D.; Matyjaszewski, K. Macromolecules 2012, 45, 78-86.
(22) Averick, S.; Simakova, A.; Park, S.; Konkolewicz, D.; Magenau, A. J. D.; Mehl, R. A.; Matyjaszewski, K. ACS Macro Lett. 2012, 1, 6-10.
(23) Braunecker, W. A.; Matyjaszewski, K. Progress in Polymer Science 2007, 32, 93-146.
(24) Goto, A.; Fukuda, T. Progress in Polymer Science 2004, 29, 329-385.
(25) Grover, G. N.; Maynard, H. D. Curr. Opin. Chem. Biol. 2010, 14, 818-827.
(26) Sumerlin, B. S. ACS Macro Lett. 2012, 1, 141-145.
