Hyperbranched Polymers:
As noted above ill-defined stars were prepared from hyperbranched cores prepared by ATRP of AB* molecules, i.e. monomers that also contain an active halogen atom.(1-3) The homopolymerization of p-(chloromethyl)styrene (4) using ATRP was the first example of providing hyperbranched polymers by a controlled radical polymerization process using commercially available monomers.
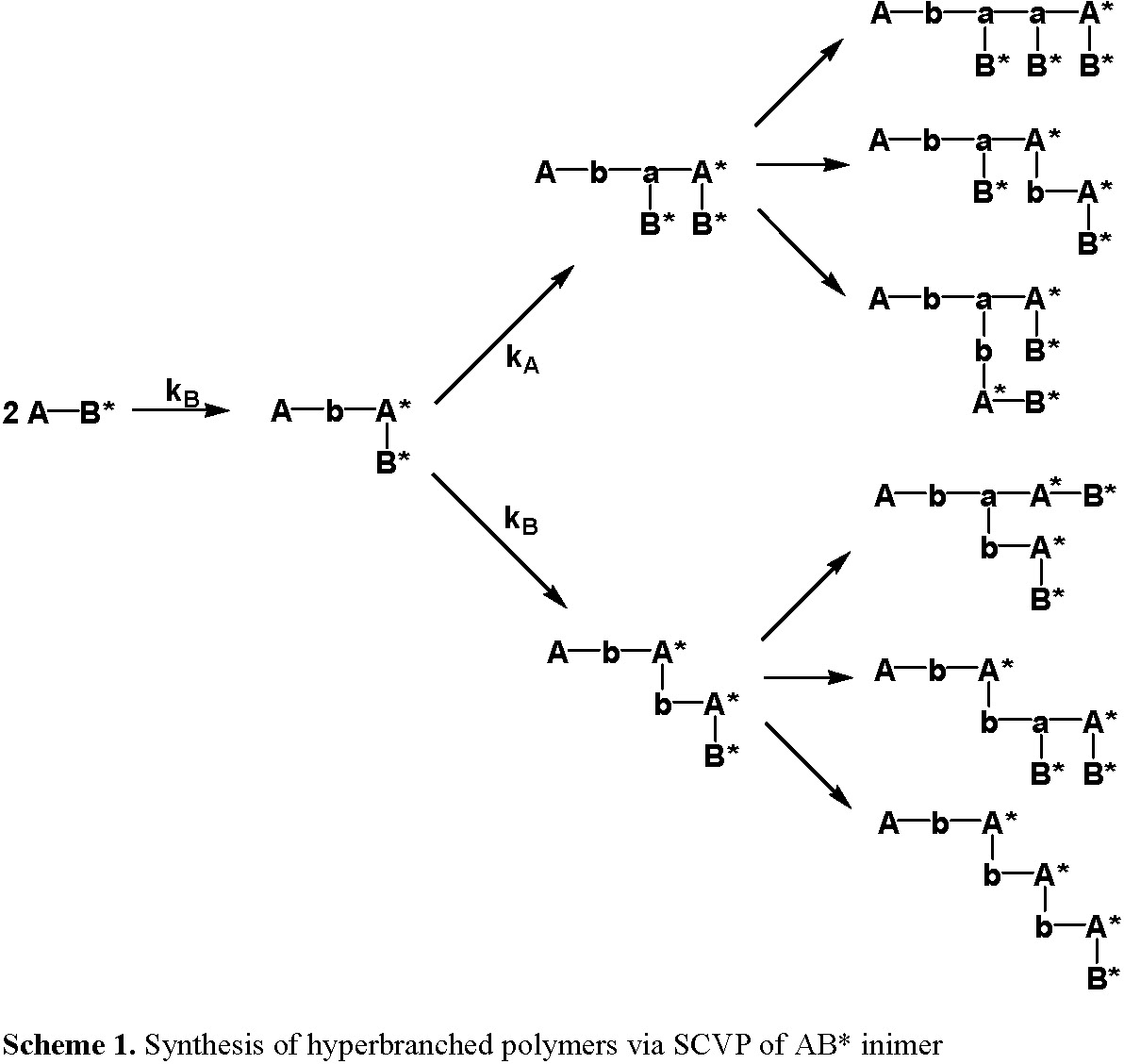
In an ideal case, depicted above, during the polymerization, the fragment B* can be converted to an active initiating group by an external stimulus and react with the chain-end vinyl group to generate a hyperbranched polymer containing a "T" shaped cross-linkage, with three radiating polymer chains and in the absence of any chain transfer and termination reactions, the number of initiating sites in a hyperbranched molecule is equal to its DP, while the number of pendant vinyl group is only one. Moreover, every primary chain contains one cross-linkage or two cross-linking units, and there is no distribution of cross-linkage among different chains. Therefore, no gelation occurs during the SCVP reaction even at complete conversion. The polymers are highly functionalized, with the number of end functionalities equal to the number of repeat units in the polymer.
Copolymers of p-(chloromethyl)styrene with styrene formed a branched polymer with a viscosity lower than a linear analog of similar molecular weight. However the hyperbranched structure was not well defined. The reason for poor definition of the core was that the structure of the branch distribution in the hyperbranched molecule depended on the catalyst employed; i.e. the (KATRP) for the ATRP, and hence the number of monomer units added during each activation cycle.(5) The concentration of CuII in the reaction medium was affected by solvent, the ligand used to complex copper, and temperature. The influence of these variables on the resulting chain architecture was studied. Improved deactivation leads to a more compact structure.(6) Use of a polymeric AB* macromolecule leads to a hyperbranched or highly branched structure with a rather low PDI, 2.6 and a significantly lower value for the Mark-Houwink exponent than a linear molecule.(7)
A review article noted the importance of controlling the polymer structure when designing functional materials.(1)
Another route to hyperbranched macromolecules is the copolymerization of a monomer with a divinyl monomer. For example, Sherrington(8) and Armes(9) used ATRP for the synthesis of branched polymers by copolymerization of methacrylate monomer and dimethacrylate cross-linker at a mole ratio close to 1:1.
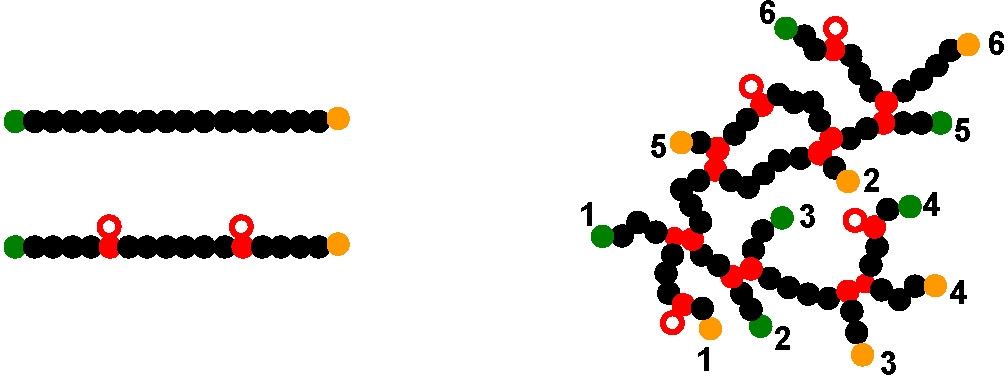
During the copolymerization the divinyl copolymer is incorporated into a growing polymer backbone generating a pendant vinyl group, lower left hand schematic, that can then be incorporated into a second growing chain to form a four arm crosslinkage. The schematic on the right hand side shows one possible structure for a branched molecule with six reacted crosslinkage units, thereby incorporating six growing chains into one molecule with six residual functional initiators and six active chain ends. Four additional pendant vinyl groups for providing further opportunities to generate additional branch points. Highly branched polymers only occurred at high monomer conversions and it is essential to keep the molar ratio of cross-linker to initiator less than 1 in order to obtain branched sols instead of gels.(10-12)
REFERENCES
(1) Matyjaszewski, K. Polymer International 2003, 52, 1559-1565.
(2) Matyjaszewski, K.; Miller, P. J.; Pyun, J.; Kickelbick, G.; Diamanti, S. Macromolecules 1999, 32, 6526-6535.
(3) Angot, S.; Murthy, K. S.; Taton, D.; Gnanou, Y. Macromolecules 1998, 31, 7218-7225.
(4) Gaynor, S. G.; Edelman, S.; Matyjaszewski, K. Macromolecules 1996, 29, 1079-1081.
(5) Yan, D.; Mueller, A. H. E.; Matyjaszewski, K. Macromolecules 1997, 30, 7024-7033.
(6) Yoo, S. H.; Yoon, T. H.; Jho, J. Y. Macromol. Rapid Commun. 2001, 22, 1319-1324.
(7) Cheng, G.; Simon, P. F. W.; Hartenstein, M.; Muller, A. H. E. Macromol. Rapid Commun. 2000, 21, 846-852.
(8) Bouhier, M.-H.; Cormack, P. A. G.; Graham, S.; Sherrington, D. C. Journal of Polymer Science, Part A: Polymer Chemistry 2007, 45, 2375-2386.
(9) Bannister, I.; Billingham, N. C.; Armes, S. P.; Rannard, S. P.; Findlay, P. Macromolecules 2006, 39, 7483-7492.
(10) Gao, C.; Muthukrishnan, S.; Li, W.; Yuan, J.; Xu, Y.; Mueller, A. H. E. Macromolecules 2007, 40, 1803-1815.
(11) Gao, H.; Li, W.; Matyjaszewski, K. Macromolecules 2008, 41, 2335-2340.
(12) Gao, H.; Miasnikova, A.; Matyjaszewski, K. Macromolecules 2008, 41, 7843-7849.
