Polymerization in Mixed Solvents
Liquid/Liquid Biphasic Polymerization:
Other liquid/liquid biphasic systems have been evaluated. The simplest is a mixed solvent/water polymerization medium.(1) A mixture of toluene and water was used as the reaction medium in the polymerization. This system was developed to take advantage of the higher solubility of water in toluene at higher temperatures and the preferential partitioning of copper halides into the aqueous phase in heterogeneous systems which results in almost complete migration of CuII into the aqueous phase after the reaction is complete and the catalyst has deliberately been oxidized. The reaction was conducted at higher temperatures in a homogenous polymerization medium then after the reaction was complete the reaction medium and catalyst complex was exposed to air and the copper migrated to the aqueous phase that separated from the toluene solvent when the reaction medium was cooled to room temperature. The amount of residual copper in the organic phase, measured by inductively coupled plasma, was less than 1 ppm.
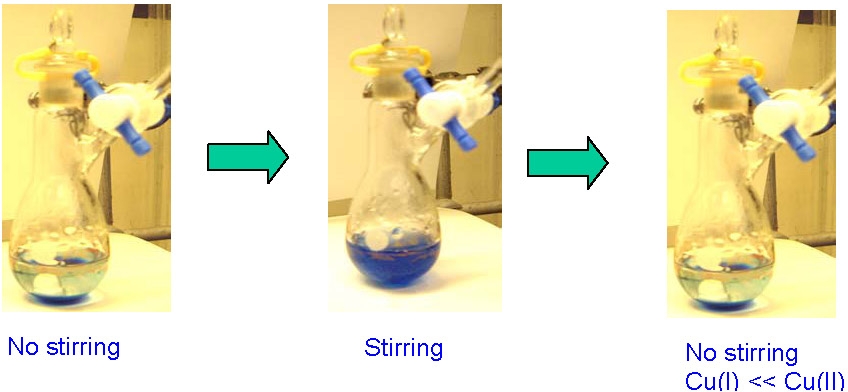
This could be converted into a continuous batch polymerization system if the oxidized CuII catalyst complex was transformed back to the activator CuI complex by a reducing agent, cf. AGET and ARGET ATRP, when a second batch of monomer/solvent is added to the flask.
The ATRP of styrene in water/toluene mixtures occurred with the preservation of Br at the polymer chain end, as confirmed by successful chain extension to form block copolymers. As noted above, the inherent inability to reuse the oxidized catalyst, accepted as fact seven years ago, has now been overcome with the development of AGET ATRP or ARGET ATRP.
Another example of using preferential solvency in a phase separating biphasic solvent system was described by Bergbreiter.(2) A heptane-soluble polyisobutylene (PIB)-CuI catalyst was synthesized in a heptane/ethanol latent biphasic solvent system. The addition of water to this initially homogeneous solution produced a biphasic solvent system that separates the PIB-CuI complex from the reaction mixture as a heptane solution. This PIB-CuI complex can also be used as the catalyst for ATRP of polystyrene. Using the differential solubility of PIB and polystyrene in heptane, a PIB-complexed CuI species can be separated from the polymer and recycled for multiple uses in further polymerization reactions with good control over molecular weight and molecular weight distribution.
A biphasic catalyst system that was immediately reusable after separation from the first reaction medium was formed by preparation of a ligand that allowed the reaction to be conducted under fluorous biphasic conditions.(3)
Another liquid/liquid system that has gained general attention is the use of ionic liquids(4-9) which, in addition to allowing ATRP in an ionic liquid,(4) ultimately allowed use of much smaller amounts of the catalyst permitting a relatively easy removal of the polymer and residual monomer from the ionic liquid. The catalytic system could be reused without further treatment. In a recent paper(8) it was reported that a CuBr/N,N,N'',N''-tetraethyldiethylenetriamine (TEDETA) anchored on an imidazolium-based ionic liquid formed a catalyst that was insoluble in the mixture of MMA and toluene but could be easily dispersed in the reaction media. The polymerization was well controlled at 60 ºC producing polymers with high initiator efficiency and low polydispersity. Once the stirring was stopped, the catalyst readily settled on the bottom of the reactor as a thin liquid layer, and thus the catalyst was easily isolated from the polymer solution. After regeneration, the recycled catalysts catalyzed a second polymerization with similar or even higher activity and improved control. The residual catalyst concentrations in the polymers were in the range 50-100 ppm. Addition of a small amount of silica gel to the polymer solution could further reduce the residual catalyst concentration.
REFERENCES
(1) Sarbu, T.; Pintauer, T.; McKenzie, B.; Matyjaszewski, K. Journal of Polymer Science, Part A: Polymer Chemistry 2002, 40, 3153-3160.
(2) Bergbreiter, D. E.; Hamilton, P. N.; Koshti, N. M. Journal of the American Chemical Society 2007, 129, 10666-10667.
(3) Haddleton, D. M.; Jackson, S. G.; Bon, S. A. F. J. Am. Chem. Soc. 2000, 122, 1542-1543.
(4) Sarbu, T.; Matyjaszewski, K. Macromolecular Chemistry and Physics 2001, 202, 3379-3391.
(5) Biedron, T.; Kubisa, P. Macromol. Rapid Commun. 2001, 22, 1237-1242.
(6) Biedron, T.; Kubisa, P. Journal of Polymer Science, Part A: Polymer Chemistry 2002, 40, 2799-2809.
(7) Ma, H.; Wan, X.; Chen, X.; Zhou, Q.-F. Journal of Polymer Science, Part A: Polymer Chemistry 2002, 41, 143-151.
(8) Ding, S.; Radosz, M.; Shen, Y. Macromolecules 2005, 38, 5921-5928.
(9) Tang, H.; Tang, J.; Ding, S.; Radosz, M.; Shen, Y. Journal of Polymer Science, Part A: Polymer Chemistry 2005, 43, 1432-1443.
