Activator Generated by Electron Transfer (AGET) ATRP
AGET ATRP initiation systems are similar to SR&NI ATRP in that it starts with (pseudo)alkyl halides as initiators and transition metal complexes in their oxidatively stable state (e.g., CuIIBr2/ligand) as catalyst precursors allowing the most active catalyst complexes to be added to the reaction prior to in-situ activation. However, instead of employing a conventional radical initiator to activate the catalyst complex as in "reverse" ATRP and SR&NI, a non-radical forming reducing agent is employed to generate the activator from the higher oxidation state transition metal complex. Reducing agents such as tin 2-ethylhexanoate,(1) ascorbic acid,(2) or MAO(3) react with the oxidatively stable CuII complex and generate the activator, i.e. the transition metal complex in its lower oxidation state. Indeed, retrospectively, we can now view the work using Cu0 to provide in situ reduction of CuII(4) as an early, unrecognized precursor, of AGET ATRP.
In an AGET ATRP the Activators are Generated by Electron Transfer, without direct involvement of organic radicals capable of initiating a radical reaction or formation of molecules that can act as initiators. Therefore an additional requirement for a successful AGET ATRP is that the reducing agents should be selected so that the reduction occurs without formation of intermediates or products that could form new initiators for an ATRP. Some reducing agents can also react directly with alkyl halides but exchange reactions are too slow to provide polymerization control.
AGET ATRP has a significant advantage over "reverse" ATRP and SR&NI ATRP, described above, because it provides a route for synthesizing pure tele-functional polymeric materials of any desired architecture(1-3,5) while employing active catalyst complexes. All reagents that are directly added to the reaction are shown inred italics in the following scheme. These reagents are stable in the presence of oxygen.
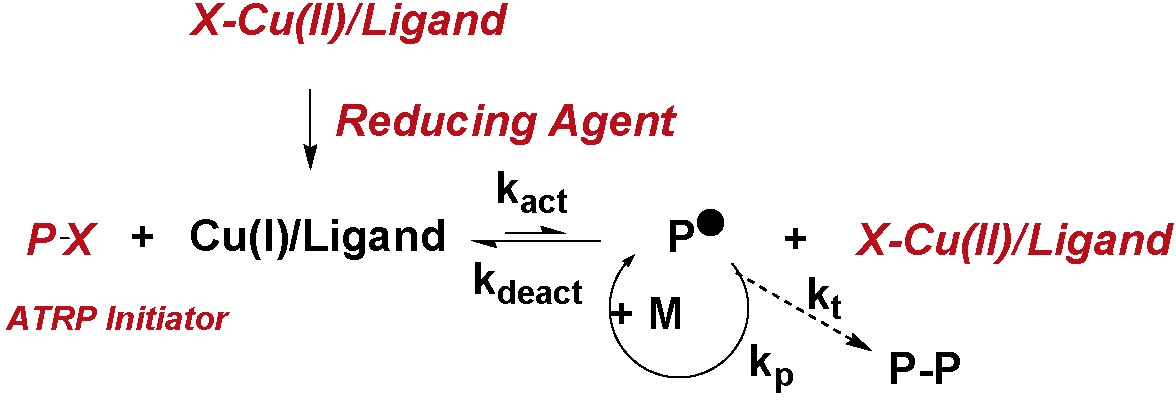
Furthermore, since AGET ATRP can be also successfully carried out in miniemulsion,(2,6,7) this is of potential commercial importance. All agents initially added to the reaction mix prior to dispersion by high shear forces, or sonication, are stable in the presence of air. The reducing agent can be added, at a controlled rate, after a stable miniemulsion is formed to provide a controlled rate of initiation, i.e. provide highly efficient initiation, and subsequently control the rate of propagation of the reaction, by continuously controlling the ratio of CuI to CuII.
An additional advantage of AGET initiation is that the reducing agent can be used to remove dissolved oxygen from the system and hence the reaction can be conducted in the presence of a limited amount of air.(1-3,5)
Furthermore it was the development of AGET ATRP that allowed extension of an ATRP micromulsion procedure which was critical to the developmentof a true scalable ab initio emulsion system,(8) and inverse miniemulsion polymerization procedures.(9)
Pure telechelic homopolymers and pure block copolymers were successfully synthesized via AGET ATRP in bulk and miniemulsion copolymerization reactions.
2-D chromatographic analysis of samples of some of the copolymers prepared by AGET ATRP (see second set of images below) clearly illustrate that we resolved the problem that provided the incentive for this development in initiating systems, i.e. the formation of a measurable fraction of homopolymer due to the action of a standard radical initiator in SR&NI systems.
2-D chromatographic analysis are particularly useful at showing the presence of minor components in a material prepared even in a well controlled CRP, note the presence of 1% of the coupled product in the following analysis as the reaction was driven to high conversion.
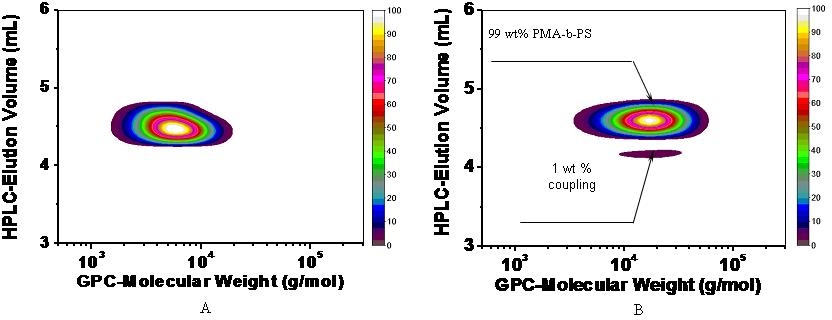
2D chromatograms of a linear PMA macroinitiator (A) and the final block copolymer PMA-b-PS (B) synthesized by AGET ATRP in miniemulsion. The 1st dimension is HPLC under the critical condition for PS, and the 2nd dimension is GPC with PS standard as calibration. [2]
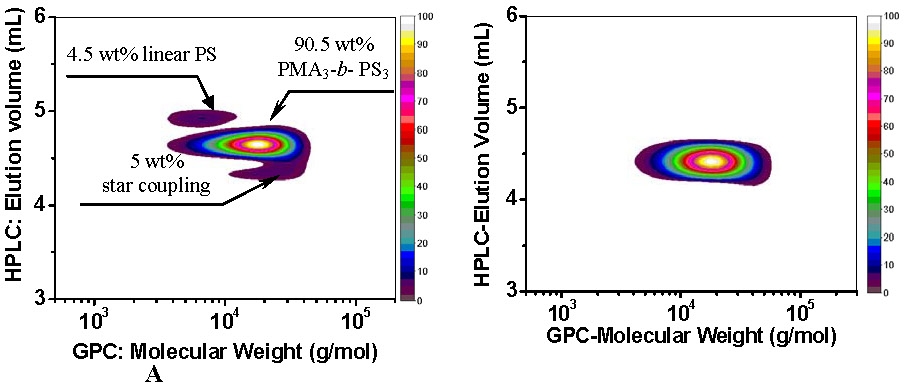
2D chromatogram of star block copolymer PMA3-b-PS3 synthesized using 3-arm trifunctional PMA macroinitiator a) by SR&NI ATRP in miniemulsion; b) by AGET ATRP in miniemulsion. [2]
These chromatograms show that AGET ATRP can produce the same spectrum of pure segmented copolymers as a normal ATRP while additionally allowing the precursors of very active catalysts to be added to the reaction in their stable oxidative state.
This procedure has now emerged as the preferred manner of initiating ATRP in a broad range of media preparing well defined materials for a number of applications,(10-18) including high-molecular-weight biocompatible brush-like polymers,(19) protein-nanogels, (20)thermally responsive copolymers,(21) reactive surfactants,(22) hybrid nanoparticles,(23) in miniemulsion and inverse miniemulsion procedures.(19, 20, 24, 25)
REFERENCES
(1) Jakubowski, W.; Matyjaszewski, K. Macromolecules 2005, 38, 4139-4146.
(2) Min, K.; Gao, H.; Matyjaszewski, K. Journal of the American Chemical Society 2005, 127, 3825-3830.
(3) Yamamura, Y.; Matyjaszewski, K. Journal of Macromolecular Science, Part A: Pure and Applied Chemistry 2007, 44, 1035-1039.
(4) Woodworth, B. E.; Metzner, Z.; Matyjaszewski, K. Macromolecules 1998, 31, 7999-8004.
(5) Matyjaszewski, K.; Bombalski, L.; Jakubowski, W.; Min, K.; Spanswick, J.; Tsarevsky, N. In PCT Int. Appl.; (Carnegie Mellon University, USA). WO 2005087819, 2005; p 96 pp.
(6) Stoffelbach, F.; Belardi, B.; Santos, J. M. R. C. A.; Tessier, L.; Matyjaszewski, K.; Charleux, B. Macromolecules (Washington, DC, United States) 2007, 40, 8813-8816.
(7) Esteves, A. C. C.; Bombalski, L.; Trindade, T.; Matyjaszewski, K.; Barros-Timmons, A. Small 2007, 3, 1230-1236.
(8) Min, K.; Gao, H.; Matyjaszewski, K. J. Am. Chem. Soc. 2006, 128, 10521-10526.
(9) Oh, J. K.; Tang, C.; Gao, H.; Tsarevsky, N. V.; Matyjaszewski, K. Journal of the American Chemical Society 2006, 128, 5578-5584.
(10) Grignard, B.; Jerome, C.; Calberg, C.; Jerome, R.; Wang, W.; Howdle, S. M.; Detrembleur, C. Macromolecules 2008, 41, 8575-8583.
(11) Kwiatkowski, P.; Jurczak, J.; Pietrasik, J.; Jakubowski, W.; Mueller, L.; Matyjaszewski, K. Macromolecules 2008, 41, 1067-1069.
(12) Lai, G.-q.; Hu, Z.-q.; Wu, L.-b.; Wu, J.-r.; Shen, Y.-j. Huadong Ligong Daxue Xuebao, Ziran Kexueban 2008, 34, 529-532.
(13) Li, W.; Min, K.; Matyjaszewski, K.; Stoffelbach, F.; Charleux, B. Macromolecules 2008, 41, 6387-6392.
(14) Siegwart, D. J.; Oh, J. K.; Gao, H.; Bencherif, S. A.; Perineau, F.; Bohaty, A. K.; Hollinger, J. O.; Matyjaszewski, K. Macromol. Chem. Phys. 2008, 209, 2179-2193.
(15) Stoffelbach, F.; Griffete, N.; Bui, C.; Charleux, B. Chem. Commun. 2008, 4807-4809.
(16) Tan, Y.; Yang, Q.; Sheng, D.; Su, X.; Xu, K.; Song, C.; Wang, P. e-Polymers 2008, No pp. given.
(17) Zhang, L.; Cheng, Z.; Shi, S.; Li, Q.; Zhu, X. Polymer 2008, 49, 3054-3059.
(18) Zhang, L.; Cheng, Z.; Tang, F.; Li, Q.; Zhu, X. Macromol. Chem. Phys. 2008, 209, 1705-1713.
(19) Oh, J. K.; Perineau, F.; Charleux, B.; Matyjaszewski, K. J. Polym. Sci., Part A: Polym. Chem. 2009, 47, 1771-1781.
(20) Averick, S. E.; Magenau, A. J. D.; Simakova, A.; Woodman, B. F.; Seong, A.; Mehl, R. A.; Matyjaszewski, K. Polym. Chem. 2011, 2, 1476-1478.
(21) Dong, H.; Matyjaszewski, K. Macromolecules 2010, 43, 4623-4628.
(22) Li, W.; Matyjaszewski, K. Macromolecules 2011, 44, 5578-5585.
(23) Li, Q.; Zhang, L.; Zhang, Z.; Zhou, N.; Cheng, Z.; Zhu, X. J. Polym. Sci., Part A: Polym. Chem. 2010, 48, 2006-2015.
(24) Averick Saadyah, E.; Paredes, E.; Irastorza, A.; Shrivats Arun, R.; Srinivasan, A.; Siegwart Daniel, J.; Magenau Andrew, J.; Cho Hong, Y.; Hsu, E.; Averick Amram, A.; Kim, J.; Liu, S.; Hollinger Jeffrey, O.; Das Subha, R.; Matyjaszewski, K. Biomacromolecules 2012, 13, 3445-3449.
(25) Li, W.; Matyjaszewski, K. Polym. Chem. 2012, 3, 1813-1819.
