ATRP from Surfaces
ATRP has been conducted from a range of surfaces since the concept was first disclosed.(1) Because of their appearance these materials have been called polymer brushes.
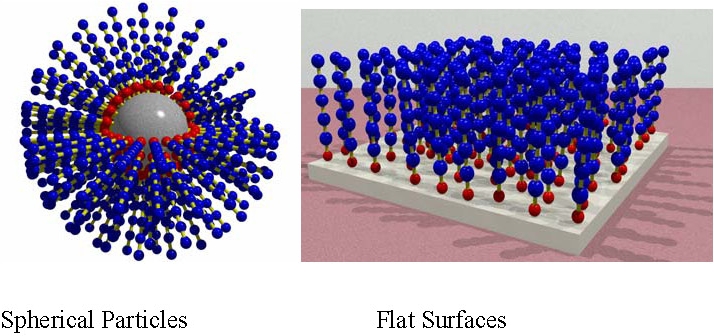
The two most common types of polymer brushes are illustrated above and have been formed by both "grafting from" and "grafting to" inorganic particles(2-8) and flat surfaces.(9-13) The synthesis of organic/inorganic hybrid materials is an area of growing interest as the useful properties of disparate components can be combined into a single material.
This section will focus on "grafting from" reactions/conditions since "grafting to"(14) requires synthesis of block or functional copolymers comprising functional groups suitable for reaction with the target substrate and will therefore be addressed in the material synthesis sections.
Controlled/"Living" Radical Polymerization (CRP) has been demonstrated to be suitable for the preparation of organic/inorganic hybrid materials with varying structural complexity on nano-, meso- and micro-scopic dimensions. ATRP has been particularly successful for synthesis of composite structures, as inorganic particles and substrates can be easily functionalized with either initiating alkyl halides or block copolymers can be synthesized with segments that can attach to inherent surface functionality. Target applications include: surfactants, elastomers, opto/magnetic materials, sensors, reinforced ultra-thin films, bio-responsive materials and patterned surfaces.
Spherical Particles
Organic/inorganic hybrid nanoparticles containing an inorganic core and tethered glassy or rubbery homopolymers or copolymers have been prepared by the ATRP of styrene and (meth)acrylates from colloidal initiators. SiO2-g-pSt hybrid nanoparticles have been prepared possessing molar masses of tethered pSt in the range of Mn = 5,000 to 33,000 g/mol and were characterized both in the solid state and in solution using respectively transmission electron microscopy (TEM) and dynamic light scattering (DLS). TEM images of SiO2-g-pSt particles revealed the formation of composite materials with interparticle spacing increasing with the increase in the molar mass of the tethered pSt chains.(6)
Comparison of hydrodynamic radii (Rh) for hybrid nanoparticles of varying size determined by DLS in toluene, versus molar masses (Mn) of pSt chains cleaved from colloids determined by SEC, revealed a linear relationship. Such linear dependence of Rh vs. Mn is a strong indication that when the particles are dispersed in toluene, the tethered chains adopt highly extended conformations, presumably due to steric interactions caused by the high grafting density and the fact that toluene is a good solvent for polystyrene.(6)
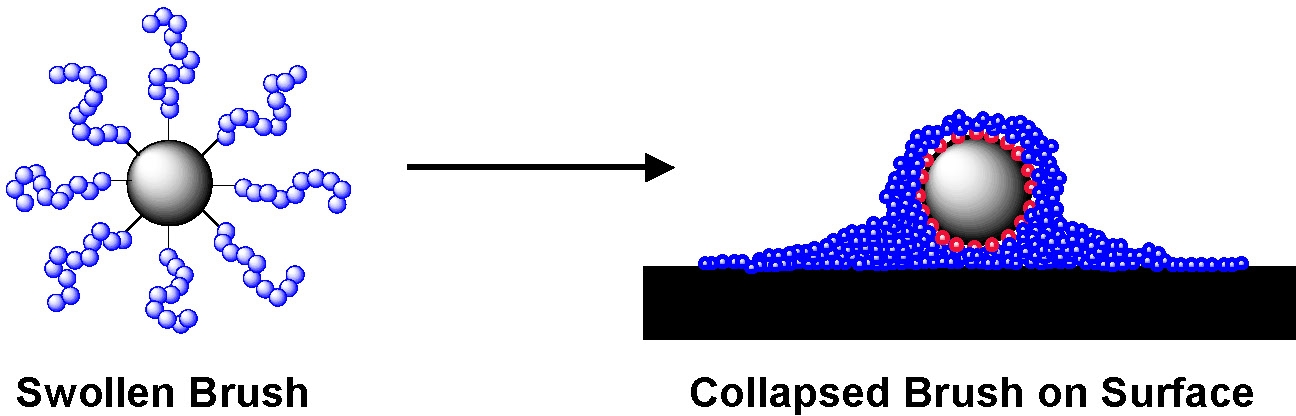
AFM examination of spherical brushes formed by grafting n-butyl acrylate from silica particles deposited on mica surfaces show that the swollen brush has collapsed and that the hard silica core is surrounded by the soft halo of tethered grafted chains.(6)
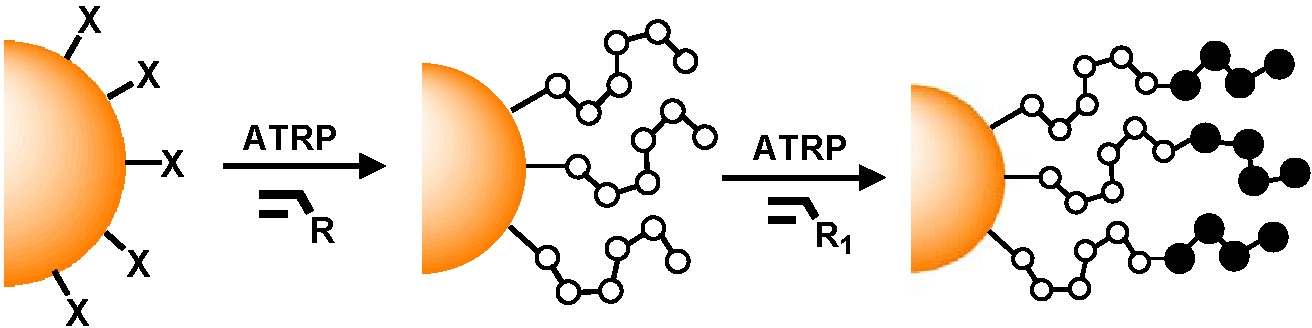
Since the polymerization from the surface is being conducted by controlled polymerization processes, it is a relatively simple task to isolate the particles and add them to a fresh monomer solution to form tethered block copolymers.
In order to minimize termination reactions and gel formation during a "grafting from" a particle surface polymerization in a "bulk" system one should add excess monomer and target a slow rate of reaction. In accordance with the persistent radical effect, adding significant levels of the deactivator to the contact solution is a convenient tool for slowing down the rate of polymerization and facilitating exchange between active radical and tethered dormant oligo/polymeric species. Often sacrificial initiator is added to the contacting reaction medium to both provide a means of following the reaction and to assist in controlling initiation from the surface.(10)
However in commercial applications this presents an additional expense since the "waste" polymer has to be removed from the contacting medium as solvent and monomer are recycled. In both ATRP and NMP control can be obtained by addition of the persistent radical to the contacting reaction medium and all polymer formed is tethered to the substrate minimizing waste.(11)
As noted one of the problems associated with use of multifunctional initiators in an ATRP is the impact of termination reactions on the properties of the final material. Termination can occur either inter-molecularly resulting in crosslinking, eventually resulting in gel formation, or intra-molecularly which only affects functionality and distribution of tethered chains. Accordingly, reaction conditions should be selected to minimize inter-particle termination reactions including:
- addition of the redox conjugate to minimize initial termination reactions that normally build up the persistent radical and to slow down propagation,
- stop the reactions at low conversions,
- conduct the reactions in dilute solution,
- or conduct the reaction in dispersed media.
Conducting the reaction in a miniemulsion also reduces the impact of inter-particle coupling and stops the formation of a macroscopic gel.(12,13)
A recent improvement in the synthesis of composite nano-particles involved the one-pot synthesis of thermally stable core/shell gold nanoparticles (Au-NPs) which was developed via surface-initiated ATRP of butyl acrylate and a dimethacrylate-based crosslinker.(15) This approach overcomes the potential problems associated with the robustness of the initial initiator/surface links. The higher reactivity of the crosslinker enables the formation of a thin crosslinked polymer shell around the surface of the Au-NP before the growth of linear polymer chains from the shell. The crosslinked polymer inner-shell served as a robust protective layer, prevented the dissociation of linear polymer brushes from the surfaces of the Au-NPs during subsequent melt fabrication processes, and provided the Au-NPs excellent thermal stability at elevated temp. (e.g., 110 0C for 24 h). This synthetic method could be easily expanded for preparation of other types of inorganic/polymer nano-composites with significantly improved stability.
Inorganic surfaces other than silica have also been used in grafting from reactions to form hybrid particles with desired properties, including flame retardant materials(16) and carbon black pigments.(8)Flat Surfaces
Modification of surfaces with thin polymeric films allows one to tailor surface properties such as wetability, biocompatibility, biocidal activity, adhesion, adsorption, corrosion resistance and friction. Polymers with reactive groups or segments can be prepared for "grafting onto" surfaces or functional groups can be attached to the surface for a more efficient "grafting from" approach. The properties of surfaces are addressed elsewhere on this site in this section we primarily address "grafting from" surface tethered initiators.
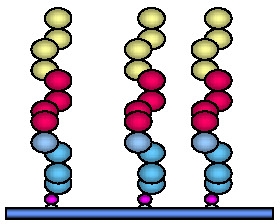
The density of the tethered initiator can be varied over a wide range. The conformation of the final tethered polymer depends on the graft density, and hence initial initiator density. Different nomenclature is currently employed to describe the result of different graft density on the topology of the tethered polymer chain:
- when the distance between attachment points (D) is significantly more than the Flory radius = aN (RF) the morphology is described as "dilute non-overlapping mushroom"; (The Flory radius RF is the average coil dimension in solution. If the solution is a good solvent for the polymer, the Flory radius scales as RF = aN0.6, where a is the size of a monomer unit.)
- when D is approximately twice RF a "weakly overlapping mushroom" is formed,
- then as the density of grafted chains exceeds 2 RF an "extended mushroom,"
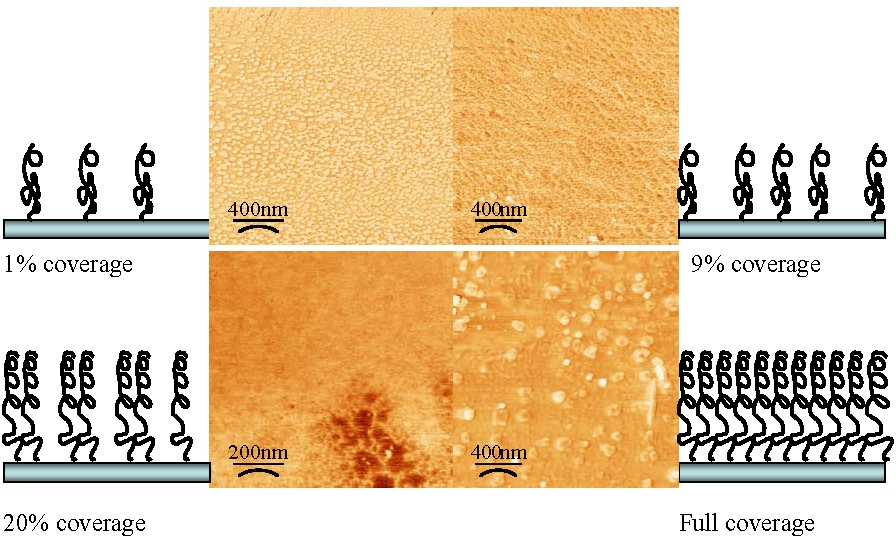
The graft density obviously affects the morphology of the tethered (co)polymer chains.
As shown above, once the initiator coverage exceeds 20% there is complete coverage of the surface with tethered chains providing a relatively smooth film with occasional defects. At lower levels of initiator coverage the grafts were increasingly patchy but at >1% coverage they formed highly uniform, isolated domains of tethered materials indicating the presence of collapsed tethered chains on the surface.
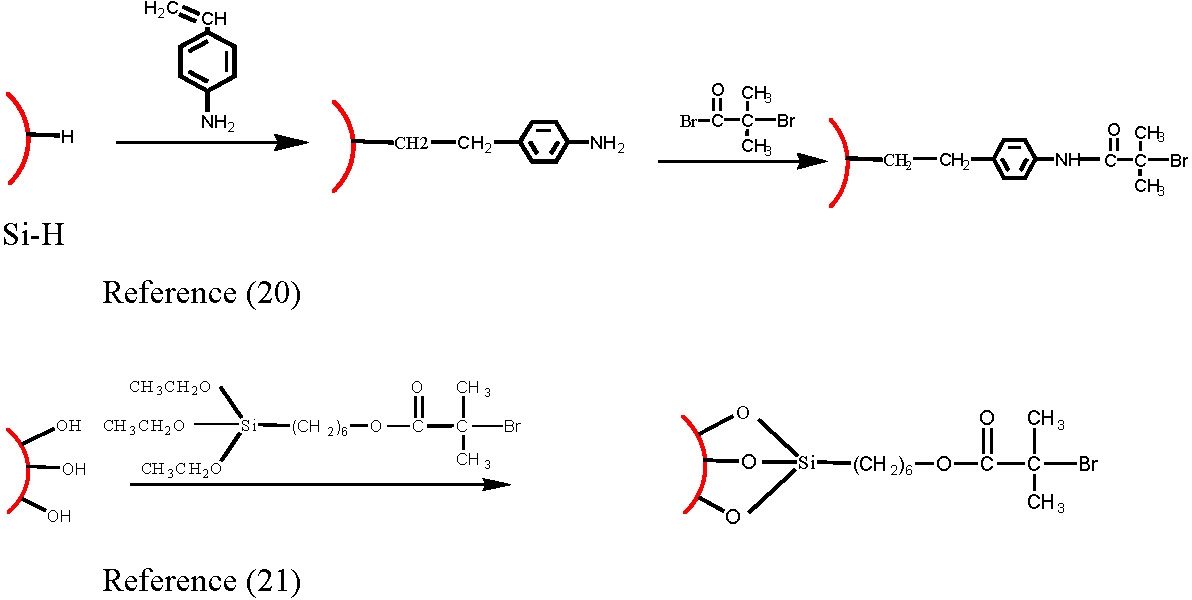
Initiators for CRP processes have been attached to a variety of surfaces including silicon, gold and carbon black, using a variety of linking groups.(8,14,17-21) The conditions noted above for grafting from particles should be applied to grafting from surfaces.
In our studies initiator density (Id) was assumed to be the same as the molar ratio of the ratio of the molecule with attached initiator functionality (nATRP) and "blank" tetherable functional molecules. Id = nATRP/(nATRP + nBlank)
Grafting for "Everyone"
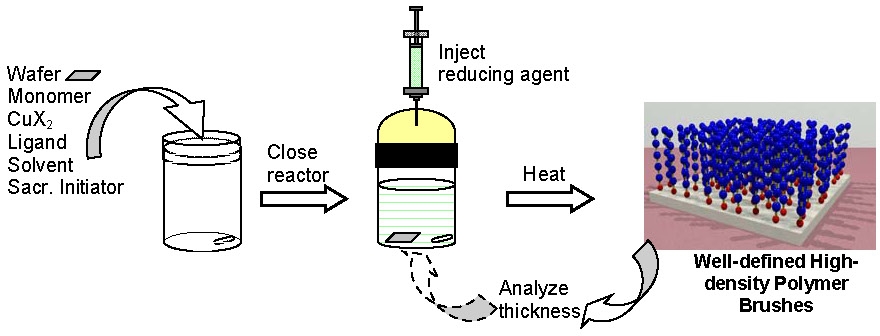
Historically an ATRP, as in any radical polymerization process, had to be carried out in rigorously deoxygenated systems to prevent trapping of propagating radicals by oxygen. However, with the development of ARGET ATRP it is now possible to conduct an ATRP in the presence of limited amounts of air with a very small (typically ppm) amount of copper catalyst together with an appropriate excess of reducing agent. This technique was successfully applied to the preparation of densely grafted polymer brushes, poly(Bu acrylate) homopolymer, and poly(Bu acrylate)-block-polystyrene copolymer from silicon wafers (0.4 chains/nm2). This simple new method of grafting well-defined polymers does not require any special equipment and can be carried out in vials or jars without deoxygenation. The grafting for "everyone technique is especially useful for wafers and other large objects and may be also applied to the synthesis of molecular hybrids and bioconjugates.(12)
REFERENCES
(1) Matyjaszewski, K.; Gaynor, S. G.; Coca, S. In U.S.P.T.O.; (Carnegie Mellon University, USA). US6541580, 2003; pp 90 pp., Cont.-in-part of U.S. 6407187.
(2) Pyun, J.; Matyjaszewski, K. Macromolecules 2000, 33, 217-220.
(3) Pyun, J.; Matyjaszewski, K.; Kowalewski, T.; Savin, D.; Patterson, G.; Kickelbick, G.; Huesing, N. J. Am. Chem. Soc. 2001, 123, 9445-9446.
(4) Pyun, J.; Xia, J.; Matyjaszewski, K. ACS Symposium Series 2003, 838, 273-284.
(5) Savin, D. A.; Pyun, J.; Patterson, G. D.; Kowalewski, T.; Matyjaszewski, K. Journal of Polymer Science, Part B: Polymer Physics 2002, 40, 2667-2676.
(6) Pyun, J.; Jia, S.; Kowalewski, T.; Patterson, G. D.; Matyjaszewski, K. Macromolecules 2003, 36, 5094-5104.
(7) Matyjaszewski, K.; Pyun, J. In PCT Int. Appl.; (Carnegie Mellon University, USA). WO0228912, 2002; p 77 pp.
(8) Liu, T.; Jia, S.; Kowalewski, T.; Matyjaszewski, K.; Casado-Portilla, R.; Belmont, J. Langmuir 2003, 19, 6342-6345.
(9) Jones, D. M.; Brown, A. A.; Huck, W. T. S. Langmuir 2002, 18, 1265-1269.
(10) von Werne, T. A.; Germack, D. S.; Hagberg, E. C.; Sheares, V. V.; Hawker, C. J.; Carter, K. R. Journal of the American Chemical Society 2003, 125, 3831-3838.
(11) Matyjaszewski, K.; Miller, P. J.; Shukla, N.; Immaraporn, B.; Gelman, A.; Luokala, B. B.; Siclovan, T. M.; Kickelbick, G.; Vallant, T.; Hoffmann, H.; Pakula, T. Macromolecules 1999, 32, 8716-8724.
(12) Matyjaszewski, K.; Dong, H.; Jakubowski, W.; Pietrasik, J.; Kusumo, A. Langmuir 2007, 23, 4528-4531.
(13) Huang, J.; Murata, H.; Koepsel, R. R.; Russell, A. J.; Matyjaszewski, K. Biomacromolecules 2007, 8, 1396-1399.
(14) Tsujii, Y.; Ohno, K.; Yamamoto, S.; Goto, A.; Fukuda, T. Advances in Polymer Science 2006, 197, 1-45.
(15) Dong, H.; Zhu, M.; Yoon Jeong, A.; Gao, H.; Jin, R.; Matyjaszewski, K. J Am Chem Soc 2008, 130, 12852-12853.
(16) Ok, J.; Matyjaszewski, K. Journal of Inorganic and Organometallic Polymers and Materials 2006, 16, 129-137.
(17) Saleh, N.; Sirk, K.; Liu, Y.; Phenrat, T.; Dufour, B.; Matyjaszewski, K.; Tilton, R. D.; Lowry, G. V. Environmental Engineering Science 2007, 24, 45-57.
(18) Pyun, J.; Jia, S.; Kowalewski, T.; Matyjaszewski, K. Macromolecular Chemistry and Physics 2004, 205, 411-417.
(19) Unsworth, L. D.; Tun, Z.; Sheardown, H.; Brash, J. L. Journal of Colloid and Interface Science 2005, 281, 112-121.
(20) Xu, F. J.; Zhong, S. P.; Yung, L. Y. L.; Kang, E. T.; Neoh, K. G. Biomacromolecules 2004, 5, 2392-2403.
(21) Ohno, K.; Morinaga, T.; Koh, K.; Tsujii, Y.; Fukuda, T. Macromolecules 2005, 38, 2137-2142.
