Incorporation of Functional Groups into Polymers Prepared by ATRP
Introduction
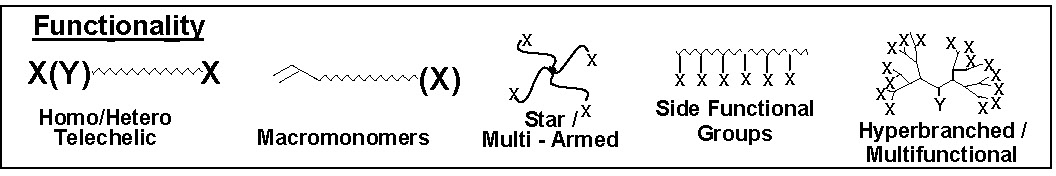
Functional groups increase the utility of polymers and are fundamental to the development of many aspects of structure-property relationships. The functionality present on the monomer units determines the solubility of the polymer in a given solvent. One can control the hydrophilicity/phobicity, or polarity of a copolymer, and the elasticity or modulus of a material by selecting appropriate monomers. End functionalized polymers are used for blend compatibilization during reactive processing and in many thermosetting compositions e.g. epoxy-functional polymers and functional materials form the basis of the majority of products prepared for dispersant, coating, adhesive, and sealant, etc. applications.
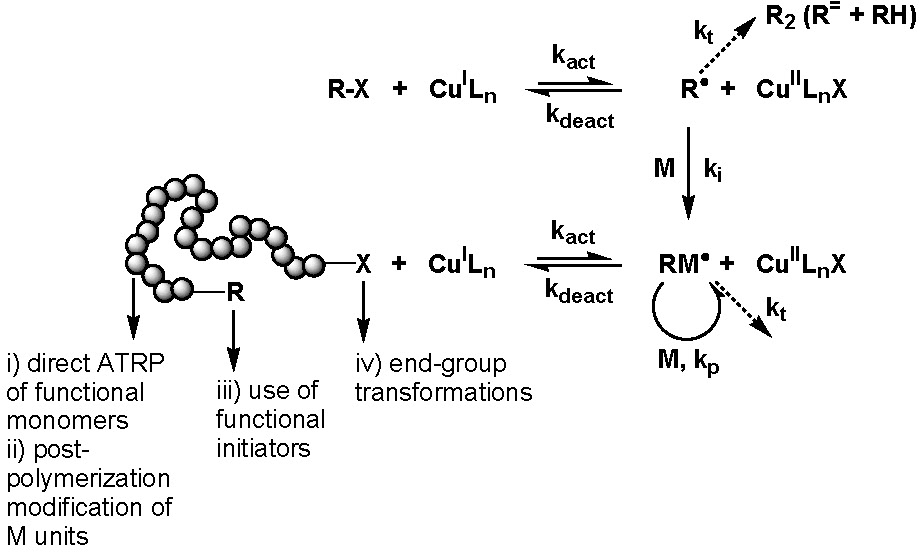
Four synthetic strategies can be employed for the synthesis of well-defined polymers with site specific functional groups using ATRP.(1)
They are:
i) direct polymerization of functional monomers
ii) post-polymerization modification of monomer units
iii) use of functional ATRP initiators
iv) end-group transformation chemistry.
These approaches are summarized in the following scheme.
An obvious approach to functional materials is the direct polymerization, or copolymerization of a monomer containing the desired functionality:
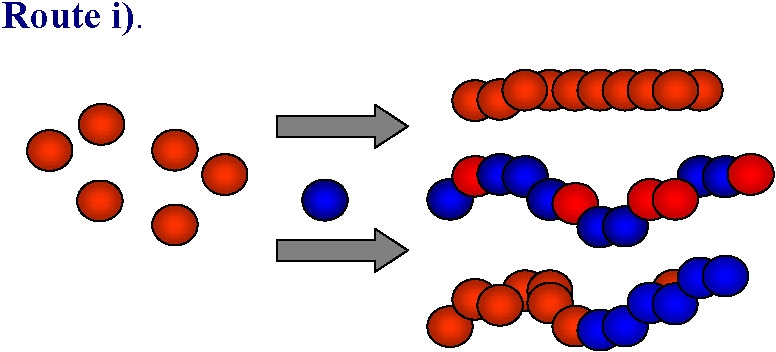
ATRP is generally tolerant of various functional polar groups and this route has been successfully used in many instances to form homopolymers, in addition to random or gradient copolymers with controlled distribution of functional monomer units along the backbone, or in specific segments of block copolymers. However in some cases, especially when strongly coordinating basic, nucleophilic or acidic monomers are used the monomers can react with either the ATRP catalyst (leading to its partial or complete deactivation) or with either the alkyl halide-type initiator or dormant polymeric species killing the active chain ends. While this limitation is being addressed by continued research targeting stable catalyst complexes the current synthetic strategy of choice in these cases is to use monomers with "protected" groups that can be transformed into the desired functionality after the polymerization:
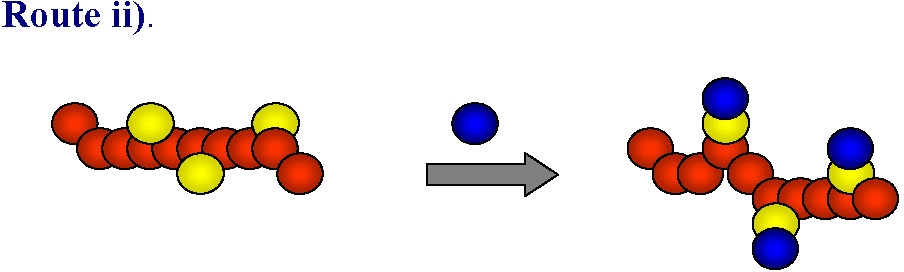
This approach has been illustrated by the preparation of well-defined polymers with distributed tetrazole groups.(2) Indeed "Click" chemistry-type post-polymerization modifications have been used to introduce various functional groups into polymers prepared by ATRP (see below).(3)
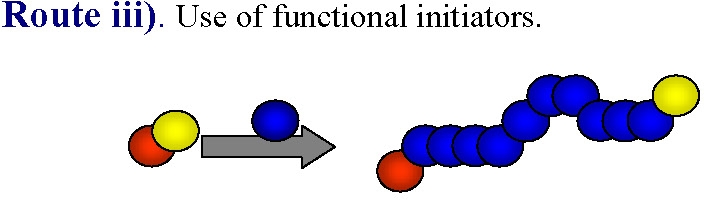
The most obvious way to incorporate a single functionality at the polymer chain end is the use of functional initiators.
This approach can be used to prepare either homo- or hetero-telechelic polymers. Use of a mono-functional initiator with desired functionality leads to direct α-functionalization of the polymer and no post-polymerization modification is required. In the vast majority of ATRP reactions the active, or growing, polymer chains are halogen-terminated, and can be further used as macroinitiators in chain-extension reactions or the initial functionality can be viewed as precursors of ω-end-functionalized polymers through end group transformations, route iv).
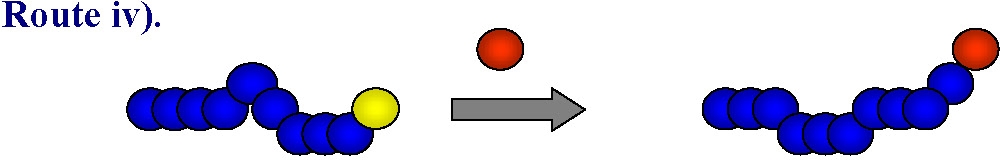
A number of nucleophilic substitution reactions have been employed to achieve this goal of post-polymerization functionalization making ATRP an attractive technique for the synthesis of well-defined end-functionalized polymers. This topic has been the subject of several detailed review articles.(4-8)
REFERENCES
(1) Tsarevsky, N. V.; Matyjaszewski, K. ACS Symposium Series 2006, 937, 79-94.
(2) Tsarevsky, N. V.; Bernaerts, K. V.; Dufour, B.; Du Prez, F. E.; Matyjaszewski, K. Macromolecules 2004, 37, 9308-9313.
(3) Golas, P. L.; Tsarevsky, N. V.; Matyjaszewski, K. Macromol. Rapid Commun. 2008, 29, 1167-1171.
(4) Coessens, V.; Pintauer, T.; Matyjaszewski, K. Prog. Polym. Sci. 2001, 26, 337-377.
(5) Davis, K. A.; Matyjaszewski, K. Advances in Polymer Science 2002, 159, 2-166.
(6) Braunecker, W. A.; Matyjaszewski, K. Progress in Polymer Science 2007, 32, 93-146.
(7) Tsarevsky, N. V.; McCarthy, P.; Jakubowski, W.; Spanswick, J.; Matyjaszewski, K. NSTI Nanotech, Nanotechnology Conference and Trade Show, Technical Proceedings, Boston, MA, United States, June 1-5, 2008, 2, 665-668.
(8) Matyjaszewski, K.; Tsarevsky, N. V. Nature Chemistry 2009, 1, 276-288.
