Initiators Containing Radically Transferable Groups
As noted in the discussion of ICAR ATRP simulations confirmed that the rate of polymerization in ICAR is governed by the rate of free radical initiator decomposition (as in RAFT) while the degree of control is ultimately determined by KATRP and the rate of deactivation (as in ATRP).(1-4) This similarity in kinetic behavior is removing the clear distinction between the three classic CRP systems, particularly when the fact that ATRP can be conducted with an expanding range of radically transferable groups, initially exemplified by the use of azide(5) and thiocyanates(6,7) as transferable groups, but now expanded to include alkyl pseudohalides based on dithioesters,(8) xanthates, dithiocarbonates or trithiocarbonates.(9-13)
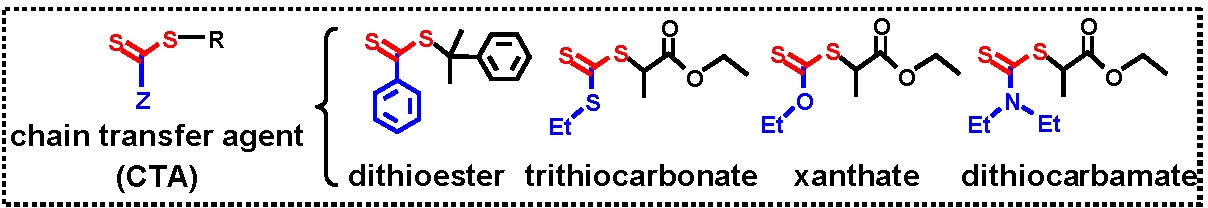
Similar to RAFT when conducting an ATRP with an initiator containing an alkyl pseudohalide as the transferable group some selection of the initiator/degenerative transfer agent has to occur.(13)
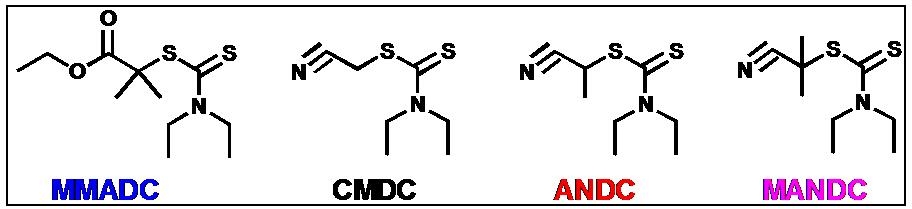
For example ATRP of styrene and MMA were successfully conducted with various alkyl diethyldithiocarbamate (DC) initiators in the presence of copper catalysts with nitrogen-based ligands.(13) EMADC, ANDC and MANDC were successful initiators for ATRP of styrene using CuBr/PMDETA as catalyst while only MANDC provided a controlled polymerization of MMA. Well-controlled polymerizations with narrow molecular weight distribution (Mw/Mn < 1.1 (St) and Mw/Mn <1.2 (MMA)) were achieved. The polymerization rate followed first-order kinetics with respect to monomer conversion, and the MW of the polymers increased linearly up to high conversion. The results of 1H NMR analysis of low-mass model compounds and chain extension from the macroinitiators confirmed that well-defined polystyrene bearing a alkyl pseudohalide as the active chain end was obtained via ATRP of St with an initiator containing an alkyl pseudohalide as the radically transferable group.
Ligand structure and ligand/copper ratio also affected the degree of control attained in the polymerization. Activation rate constants and equilibrium constants for ATRP with initiators containing alkyl pseudohalides as the radically transferable group and copper complexes were determined and the results of cyclic voltammetry with the CuIIDTA2 complex indicated that it has higher negative reduction potential and, consequently, higher (pseudo)halidophilicity than those of CuIIBr2 or CuIICl2 with the same ligand, Me6TREN. An advantage of initiating an ATRP with an alkyl pseudohalide as the transferable group, compared to a RAFT reaction with a similar transfer agent, is that no new chains are formed by added radical initiators and higher molecular weight copolymers can be prepared.
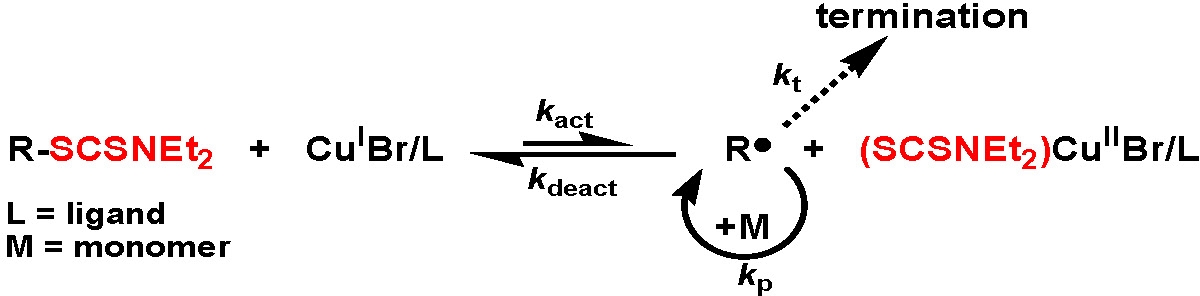
Proposed Mechanism:
There is the possibility to conduct both ATRP and RAFT reactions concurrently(12) or sequentially(14,15) with ATRP initiators containing selected alkyl pseudohalides as one of the transferable groups. As shown in the following schematic the contribution of both reaction mechanisms to chain growth is dependent on monomer, structure of the alkyl pseudohalide initiator/RAFT agent and catalyst used.
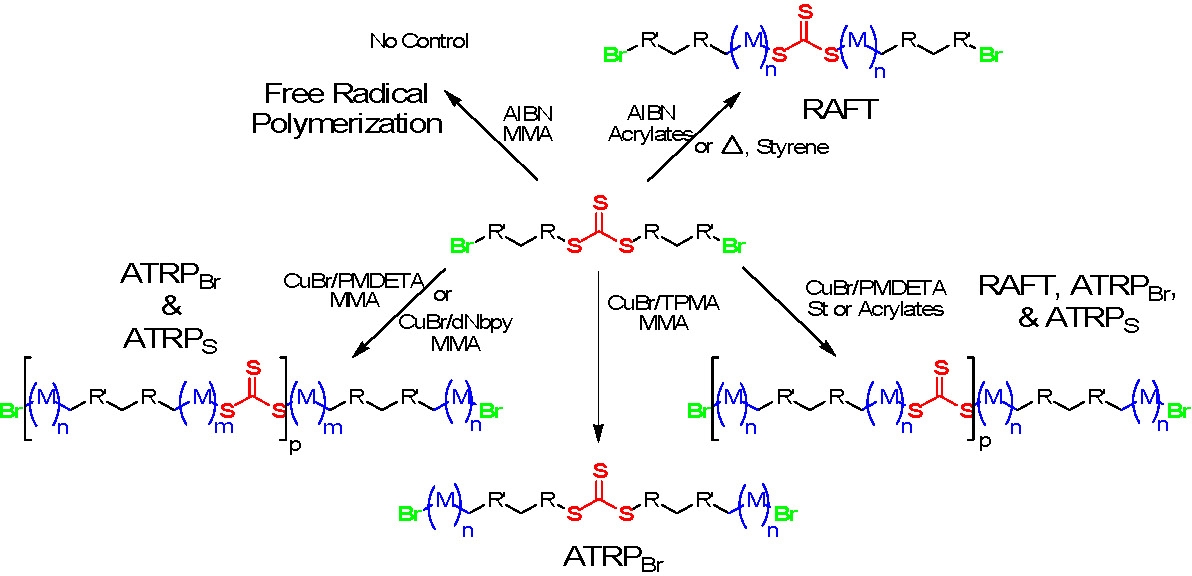
Effect of Monomer and Catalyst on Polymerization Mechanism with DiBrTTC:
In the case of concurrent CRP overall control is provided by a dual mediating system where activation/deactivation is conducted by ATRP in conjunction with degenerative exchange by RAFT. A concurrent ATRP/RAFT process with cumyl dithiobenzoate acting as the initiator/dormant species for ATRP and also as the chain transfer agent for RAFT was successfully applied to well controlled polymerization of both styrene and methy methacrylate. The polymerization displayed first-order kinetics with respect to monomer conversion and polymer molecular weight increased linearly up to high conversion.
Increasing the amount of CuBr/ligand added to the reaction resulted in faster polymerization with the rate observed in the presence of a catalyst complex formed with Me6TREN as ligand being faster than with PMDETA ligand. The rate could be further increased by adding Cu powder to the reaction to reduce the concentration of Cu(II) formed by termination reactions. Note that the contributions of both RAFT and ATRP depend on the performance of the initiator with an alkyl pseudohalide as the radically transferable group and the activity of the copper/ligand catalyst complex.
Macroinitiator (entry MI 1-2) and PMMA-b-PSt synthesis (entry BL 1-2)a
|
entry |
M/eq. |
CTA |
V-40 |
CuBr/L/Cu(0) |
t / h |
conv / % |
Mn |
Mw/Mn |
|
MI 1 |
MMA/80 |
CDB |
0.5 |
- |
16.5 |
97.0 |
8800 |
1.22 |
|
MI 2b |
MMA/80 |
CDB |
- |
2 / 4 / 0 |
16.5 |
93.2 |
8300 |
1.23 |
|
BL 1 |
St/1000 |
MI 1 |
2 |
- |
20.0 |
50.1 |
40,900 |
1.32 |
|
BL 2c |
St/1000 |
MI 2 |
- |
20/30/10 |
20.0 |
48.4 |
50,600 |
1.20 |
aAll polymerizations were performed at 80 oC. b L = bpy. c L = Me6TREN.
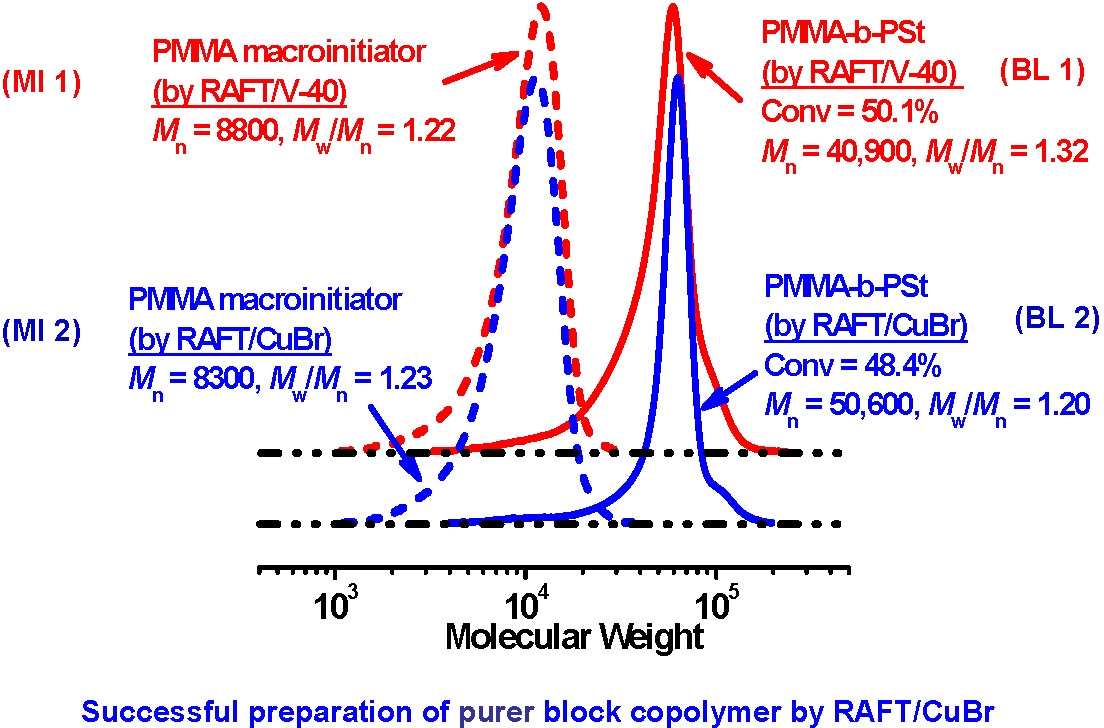
Purer block copolymers were obtained by the concurrent CRP method than with RAFT alone, demonstrating that the overall level of control in an ATRP with an initiator with an alkyl pseudohalide as the radically transferable group provides a more controlled polymerization process in comparison with conventional RAFT polymerization.
The procedure has been adapted to provide a simple and efficient synthesis of RAFT chain transfer agents via an extension of atom transfer radical coupling reactions (A) named atom transfer radical addition fragmentation, (B).(16)
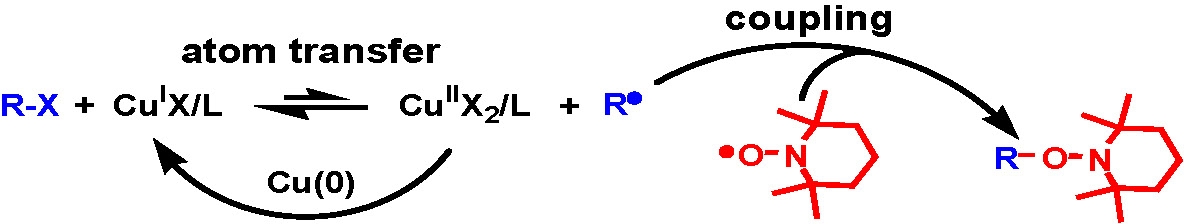
A) Atom transfer radical coupling (ATRC) for alkoxyamine synthesis:
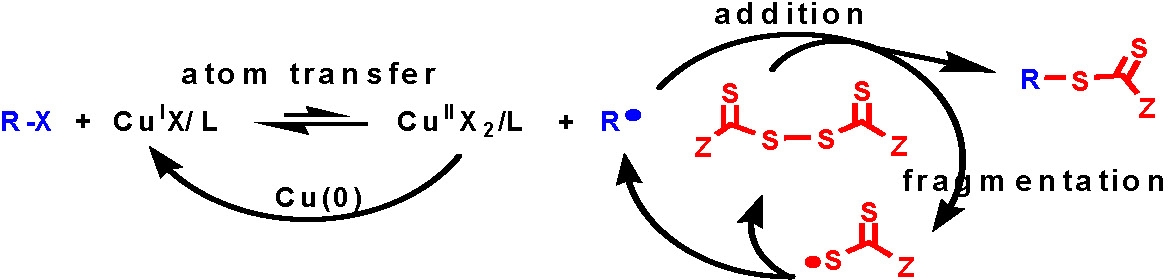
B) Atom transfer radical addition-fragmentation (ATRAF) for (RAFT) chain transfer agent synthesis:
Since RAFT lacks a universal transfer agent this latter innovation provides a simple procedure for the preparation of monomer specific RAFT agents.
REFERENCES
(1) Tang, W.; Tsarevsky, N. V.; Matyjaszewski, K. Journal of the American Chemical Society 2006, 128, 1598-1604.
(2) Matyjaszewski, K.; Jakubowski, W.; Min, K.; Tang, W.; Huang, J.; Braunecker, W. A.; Tsarevsky, N. V. Proceedings of the National Academy of Sciences of the United States of America 2006, 103, 15309-15314.
(3) Mueller, L.; Jakubowski, W.; Tang, W.; Matyjaszewski, K. Macromolecules 2007, 40, 6464-6472.
(4) Tang, W.; Matyjaszewski, K. Macromol. Theory Simul. 2008, 17, 359-375.
(5) Matyjaszewski, K.; Tsarevsky, N. In PCT Int. Appl.; (Carnegie Mellon University, USA). WO, 2002; p 64 pp.
(6) Davis, K. A.; Matyjaszewski, K. Journal of Macromolecular Science, Pure and Applied Chemistry 2004, 41, 449-465.
(7) Singha, N. K.; Klumperman, B. Macromol. Rapid Commun. 2000, 21, 1116-1120.
(8) Kabachii, Y. A.; Kochev, S. Y.; Valetskii, P. M. Vysokomolekulyarnye Soedineniya, Seriya A i Seriya B 2006, 48, 353-358.
(9) Li, P.; Qiu, K.-Y. Living and Controlled Polymerization: Synthesis, Characterization and Properties of the Respective Polymers and Copolymers 2006, 39-50.
(10) Li, P.; Qiu, K.-Y. Macromolecular Rapid Communications 2002, 23, 1124-1129.
(11) Nicolay, R.; Kwak, Y.; Matyjaszewski, K. Macromolecules 2008, 41, 4585-4596.
(12) Kwak, Y.; Nicolay, R.; Matyjaszewski, K. Macromolecules 2008, 41, 6602-6604.
(13) Kwak, Y.; Matyjaszewski, K. Macromolecules 2008, 41, 6627-6635.
(14) Nicolay, R.; Kwak, Y.; Matyjaszewski, K. Chemical Communications 2008, 5336-5338.
(15) Tong, Y.-Y.; Dong, Y.-Q.; Du, F.-S.; Li, Z.-C. Macromolecules 2008, 41, 7339-7346.
(16) Kwak, Y.; Nicolay, R.; Matyjaszewski, K. Macromolecules 2009, 42, 3738-3742.
