SARA ATRP or SET LRP?
The timeline shown below summarizes the evolution of the various mechanistic aspects of the procedure named Supplemental Activation Reducing Agent (SARA) ATRP (based on the roles of Cu0 in the overall activation and reactivation mechanism) that were disclosed prior to the reaction comprising the same reagents but named single-electron transfer living radical polymerization (SET-LRP) and the extensive detailed mechanistic and kinetic studies that confirmed the SARA ATRP mechanism. The (bracketed) numbers on the timeline are reference numbers
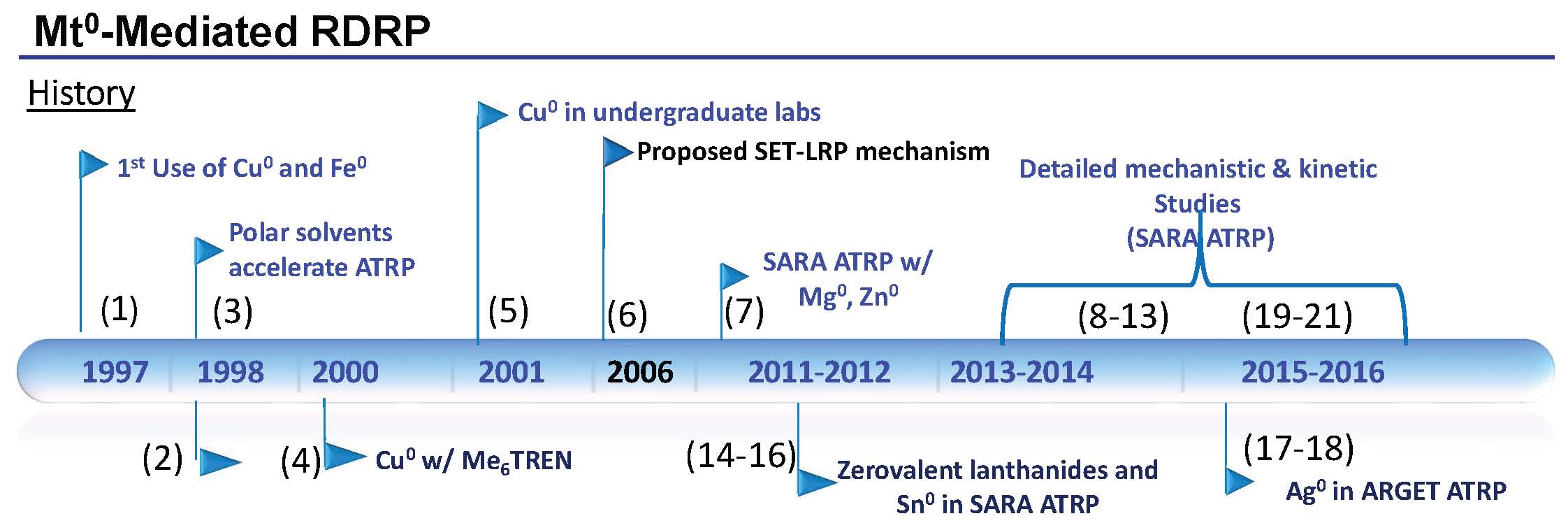
The timeline, shown above, indicates that Cu0 (and Fe0) were used for the first time in an ATRP in 1997. (1) At that time the zero oxidation state metals were employed both to directly activate alkyl halide initiators as a supplemental activator (SA) and to reduce added CuII catalyst complexes (RA) to form the CuI activator in situ in a comproportionation reaction. The zero oxidation state transition metal remained in the reaction medium to reduce the concentration of CuII formed by termination reactions and increase the rate of polymerization. In addition, both Cu0 and Fe0 were employed as the sole source of the transition metal that formed the transition metal complexes required for an ATRP in the presence of excess ligand and an initiator.
Me6TREN was used as a ligand for Cu based ATRP in 1998. (2) The active catalyst complex allowing the polymerization of acrylates at ambient temperture.
The role of polar solvents in increasing the rate of polymerization was also discussed at that time. (3)
A paper in 2000 described optimization of an ATRP with a copper bromide complex with one equivalent of Me6TREN at low concentrations of catalyst in the presence of copper powder in the reaction mixture for the preparation of well-defined poly(butyl acrylate) in bulk. (4)
The reaction set-up was so simple it was introduced in undergraduate laboratories. (5)
However, in 2006 a paper from the Percec group (6) claimed an “Ultrafast Synthesis of Ultrahigh Molar Mass Polymers by Metal-Catalyzed Living Radical Polymerization of Acrylates, Methacrylates, and Vinyl Chloride Mediated by SET at 25 DegC”. This process, named SET-LRP, was based on the contention that “polar solvents, such as H2O, alcohols, dipolar aprotic solvents, ethylene and propylene carbonate, and ionic liquids result in the instantaneous disproportionation of CuIX into Cu0 and CuIIX2 species in the presence of a diversity of N-containing ligands. This instantaneous disproportionation facilitated an ultrafast LRP in which the free radicals are generated by reaction of “nascent” and extremely reactive Cu0 species, while their deactivation is mediated by the CuIIX2 species. The activation step proceed by a low activation energy outer-sphere single-electron-transfer (SET) mechanism. The resulting SET-LRP process was activated by a catalytic amount of the electron-donor Cu0, Cu2Se, Cu2Te, Cu2S, or Cu2O species, not by CuIX.
So, even although all of the components for SET-LRP had been introduced for use in an ATRP on or before 2000 six years later the question was raised “was this procedure an ATRP with high values for KATRP or did the same reagents under the same conditions participate in a novel mechanism SET-LRP forming the same products?” The difference was attributed to the formation and high reactivity of “nascent” Cu0 (6) postulated to arise due to the instantaneous and complete disproportionation of the CuI catalyst thereby giving rise to the two active reagents in the polymerization, Cu0 and the CuII complex; the activator and deactivator.
Consequently, the mechanistic aspects of atom transfer radical polymerization in the presence of zero-valent copper and polar media have been under discussion since 2006. (7-20)
In an SET-LRP it was postulated that the alkyl halides were exclusively activated by Cu0 via an outer sphere electron transfer (OSET) process. (6) CuI did not activate alkyl halides, instead it underwent instantaneously disproportionation to form Cu0 and CuII. The formed Cu0 activated the dormant initiator and CuII acted as the deactivator while there was minimal comproportionation to retain a suitable concentration of the CuI activator. (21) This contention was surprising and the mechanism for reversible deactivation radical polymerization (RDRP) in the presence of Cu0 has been the subject of considerable interest since 2006 as a consequence of the two differing interpretations of the roles Cu0 and CuI catalyst complexes in the presence of polar solvents.
One proposed mechanism, named supplemental activator and reducing agent atom transfer radical polymerization (SARA ATRP) has Cu0 acting as a supplemental activator and a reducing agent with the supplemental activation occurring via an inner-sphere electron transfer process occurring during a slow activation step, while CuI acts as the predominant activator of the dormant alkyl halides. This occurs in the presence of a relatively slow comproportionation reaction between Cu0 and CuII and an even slower disproportionation of CuI to Cu0 and CuII. (22) In SARA ATRP there is slow activation of alkyl halides by Cu0 and comproportionation of Cu0 with CuII that compensates for the small number of radicals lost to termination reactions. On the other hand, SET-LRP assumes that the CuI species does not activate dormant alkyl halides, but undergoes instantaneous disproportionation forming Cu0 and CuII. The Cu0 activates alkyl halides through a fast outer sphere electron transfer (OSET) reaction between alkyl halides and the formed ‘nascent’ Cu0, while the formed CuII deactivates the propagating radical, as in ATRP. The combination providing an ultra-fast controlled polymerization of (meth)acrylates, and styrene. (23)
The two competing models, SARA ATRP and SET-LRP actually use exactly the same components and comprise exactly the same reactions but with vastly different contributions to the overall polymerization. The reactions involved in SARA ATRP and SET-LRP are shown in SARA or SET Scheme 1 where it can be observed that the same set of equilibria contribute to both SARA ATRP and SET-LRP, but the level of their contributions to the overall controlled polymerization process do differ dramatically.
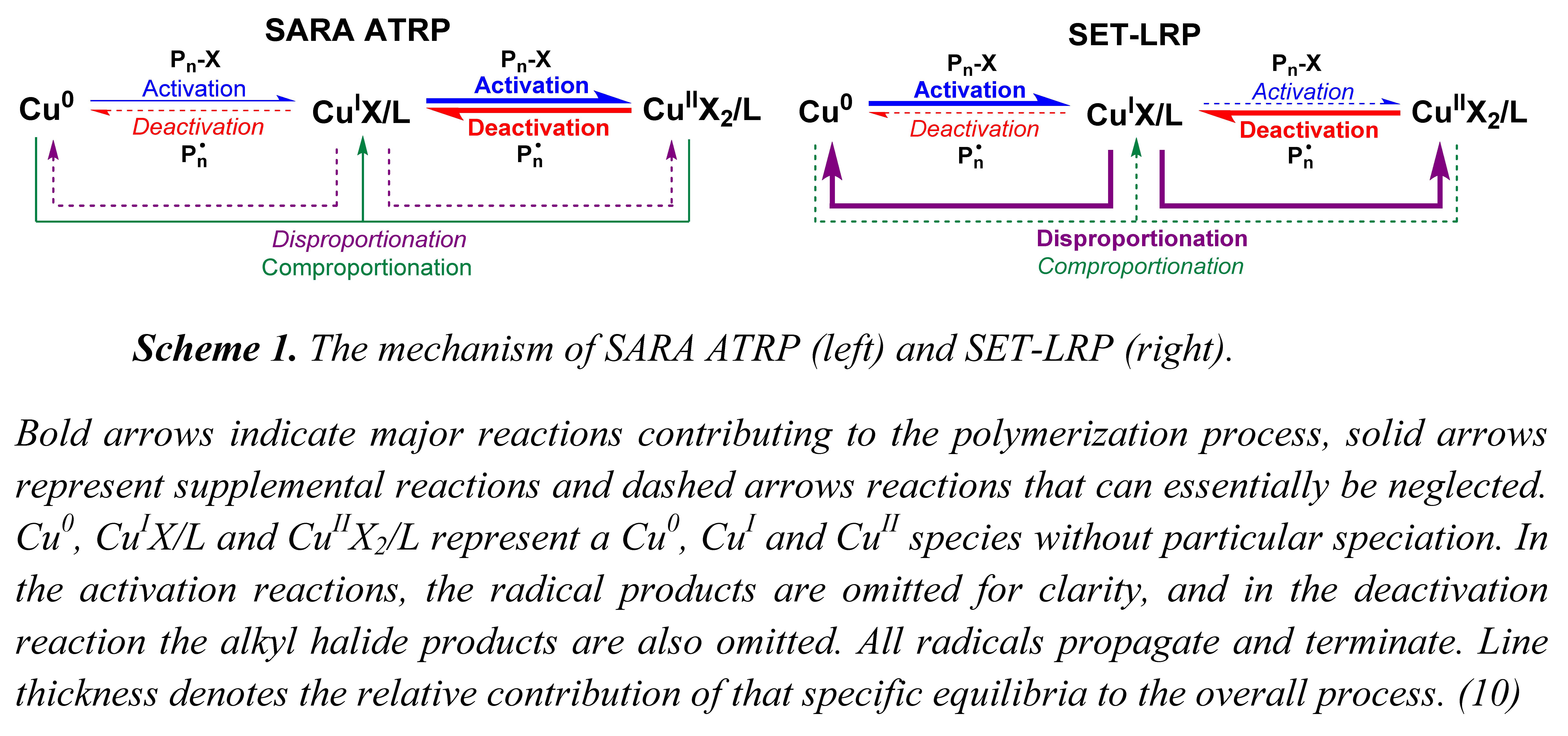
Bold arrows indicate major reactions contributing to the polymerization process, solid arrows represent supplemental reactions and dashed arrows reactions that can essentially be neglected. Cu0, CuIX/L and CuIIX2/L represent a Cu0, CuI and CuII species without particular speciation. In the activation reactions, the radical products are omitted for clarity, and in the deactivation reaction the alkyl halide products are also omitted. All radicals propagate and terminate. Line thickness denotes the relative contribution of that specific equilibria to the overall process.
The differences between SARA ATRP and SET-LRP were initially addressed in 2007 (22) when the role of Cu0 in the presence of Me6TREN in a series of solvents including DMSO, DMF and MeCN in a controlled radical polymerization was clearly defined. Activation of alkyl halides by CuI/Me6TREN is significantly faster than activation by Cu0 and disproportionation was slow in DMSO and comproportionation dominated. The role of Cu0 was to regenerate CuI and balance the ratio of [CuI] to [CuII].
However, the discussion did not end with that clarification and a new set of experiments were carried out that were designed to provide additional rate and kinetic data to model the rate of these reactions. The rate constant k, which has been determined experimentally, provides a practical route to deciding the most appropriate mechanism. The fundamentals of SARA ATRP were provided in a series of recent papers (8-11, 24) and the experimental data agrees with the SARA ATRP mechanism, since the activation of alkyl halides by CuI species is significantly faster than activation with Cu0. The activation step involves inner sphere electron transfer rather than an outer sphere electron transfer. The analysis of an extensive set of data confirms that while comproportionation is slow in DMSO, it occurs faster than disproportionation. The rate of deactivation by CuII is essentially the same as the rate of activation by CuI and the system is controlled by the classic ATRP equilibrium. The role of Cu0 in this system is to slowly and continuously supply CuI activating species and radicals, by supplemental activation and comproportionation procedures to compensate for CuI lost due to the unavoidable radical termination reactions. (12)
This conclusion was placed in a broader context of explaining unexpected data via competitive equilibria and processes in radical reactions with reversible deactivation where CuI was at the center of the competitive processes. (24) It was confirmed that even though disproportionation is thermodynamically favored over comproportionation in polar media (6) and it is relatively fast (12, 21) in highly polar media, CuI complexes are so active at interacting with alkyl halides in an ATRP and generating the CuII deactivator this pushes the CuI concentration to a very low level, which decreases the rate of disproportionation, which is proportional to [CuI]2. Consequently, disproportionation is dominated by comproportionation.
With the mechanistic understanding gained by analyzing the experimental data in the literature, the reaction conditions in SARA ATRP can be tailored towards efficient synthesis of a new generation of complex architectures and functional materials.
1. a) Matyjaszewski, K.; Coca, S.; Gaynor, S. G.; Wei, M.; Woodworth, B. E., Zerovalent Metals in Controlled/\"Living\" Radical Polymerization. Macromolecules 1997, 30 (23), 7348-7350. b) Matyjaszewski, K.; Gaynor, S. G.; Coca, S. Controlled atom or group-transfer radical polymerization, coupling of molecules, multifunctional polymerization initiators, and formation of telechelic functional material. 9840415, 19980917, 1998.
2. Xia, J.; Gaynor, S. G.; Matyjaszewski, K., Controlled/\"Living\" Radical Polymerization. Atom Transfer Radical Polymerization of Acrylates at Ambient Temperature. Macromolecules 1998, 31 (17), 5958-5959.
3. Matyjaszewski, K.; Nakagawa, Y.; Jasieczek, C. B., Polymerization of n-butyl acrylate by atom transfer radical polymerization. Remarkable effect of ethylene carbonate and other solvents. Macromolecules 1998, 31 (5), 1535-1541.
4. Queffelec, J.; Gaynor, S. G.; Matyjaszewski, K., Optimization of atom transfer radical polymerization using Cu(I)/tris(2-(dimethylamino)ethyl)amine as a catalyst. Macromolecules 2000, 33 (23), 8629-8639.
5. a) Beers, K. L.; Woodworth, B.; Matyjaszewski, K., Controlled/living radical polymerization in the undergraduate laboratories. 1. Using ATRP to prepare block and statistical copolymers of n-butyl acrylate and styrene. J. Chem. Educ. 2001, 78 (4), 544-547. b) Matyjaszewski, K.; Beers, K. L.; Woodworth, B.; Metzner, Z., Controlled/living radical polymerization in the undergraduate laboratories. 2. Using ATRP in limited amounts of air to prepare block and statistical copolymers of n-butyl acrylate and styrene. J. Chem. Educ. 2001, 78 (4), 547-550.
6. Percec, V.; Guliashvili, T.; Ladislaw, J. S.; Wistrand, A.; Stjerndahl, A.; Sienkowska, M. J.; Monteiro, M. J.; Sahoo, S., Ultrafast Synthesis of Ultrahigh Molar Mass Polymers by Metal-Catalyzed Living Radical Polymerization of Acrylates, Methacrylates, and Vinyl Chloride Mediated by SET at 25 DegC. J. Am. Chem. Soc. 2006, 128 (43), 14156-14165.
7. Zhang, Y.; Wang, Y.; Matyjaszewski, K., ATRP of Methyl Acrylate with Metallic Zinc, Magnesium, and Iron as Reducing Agents and Supplemental Activators. Macromolecules (Washington, DC, U. S.) 2011, 44 (4), 683-685.
8. Wang, Y.; Zhong, M.; Zhu, W.; Peng, C.-H.; Zhang, Y.; Konkolewicz, D.; Bortolamei, N.; Isse, A. A.; Gennaro, A.; Matyjaszewski, K., Reversible-Deactivation Radical Polymerization in the Presence of Metallic Copper. Comproportionation-Disproportionation Equilibria and Kinetics. Macromolecules 2013, 46 (10), 3793-3802.
9. Peng, C.-H.; Zhong, M.; Wang, Y.; Kwak, Y.; Zhang, Y.; Zhu, W.; Tonge, M.; Buback, J.; Park, S.; Krys, P.; Konkolewicz, D.; Gennaro, A.; Matyjaszewski, K., Reversible-Deactivation Radical Polymerization in the Presence of Metallic Copper. Activation of Alkyl Halides by Cu0. Macromolecules 2013, 46 (10), 3803-3815.
10. Zhong, M.; Wang, Y.; Krys, P.; Konkolewicz, D.; Matyjaszewski, K., Reversible-Deactivation Radical Polymerization in the Presence of Metallic Copper. Kinetic Simulation. Macromolecules 2013, 46 (10), 3816-3827.
11. Konkolewicz, D.; Wang, Y.; Zhong, M.; Krys, P.; Isse, A. A.; Gennaro, A.; Matyjaszewski, K., Reversible-Deactivation Radical Polymerization in the Presence of Metallic Copper. A Critical Assessment of the SARA ATRP and SET-LRP Mechanisms. Macromolecules 2013, 46 (22), 8749-8772.
12. Konkolewicz, D.; Krys, P.; Gois, J. R.; Mendonca, P. V.; Zhong, M.; Wang, Y.; Gennaro, A.; Isse, A. A.; Fantin, M.; Matyjaszewski, K., Aqueous RDRP in the Presence of Cu0: The Exceptional Activity of CuI Confirms the SARA ATRP Mechanism. Macromolecules 2014, 47 (2), 560-570.
13. Konkolewicz, D.; Wang, Y.; Krys, P.; Zhong, M.; Isse, A. A.; Gennaro, A.; Matyjaszewski, K., SARA ATRP or SET-LRP. End of controversy? Polym. Chem. 2014, 5 (15), 4396-4417.
14. Liu, D.; Ma, J.; Chen, H.; Yin, P.; Ji, N.; Zong, G., Single electron transfer-living radical polymerization of methyl methacrylate catalyzed by ytterbium powder. J. Polym. Sci., Part A Polym. Chem. 2011, 49 (23), 5109-5115.
15. Chen, H.; Zong, G.; Chen, L.; Zhang, M.; Wang, C.; Qu, R., Samarium powder as catalyst for SET-LRP of acrylonitrile in 1,1,1,3,3,3-hexafluoro-2-propanol for control of molecular weight and tacticity. J. Polym. Sci., Part A Polym. Chem. 2011, 49 (13), 2924-2930.
16. Hao, Z.; Chen, H.; Liu, D.; Fan, L., SET-LRP of acrylonitrile catalyzed by tin powder. J. Polym. Sci., Part A Polym. Chem. 2012, 50 (24), 4995-4999.
17. Williams Valerie, A.; Ribelli Thomas, G.; Chmielarz, P.; Park, S.; Matyjaszewski, K., A silver bullet: elemental silver as an efficient reducing agent for atom transfer radical polymerization of acrylates. J Am Chem Soc 2015, 137 (4), 1428-31.
18. Williams, V. A.; Matyjaszewski, K., Expanding the ATRP Toolbox: Methacrylate Polymerization with an Elemental Silver Reducing Agent. Macromolecules 2015, 48 (18), 6457-6464.
19. Krys, P.; Ribelli, T. G.; Matyjaszewski, K.; Gennaro, A., Relation between Overall Rate of ATRP and Rates of Activation of Dormant Species. Macromolecules 2016, 49, 2467-2476.
20. Whitfield, R.; Anastasaki, A.; Nikolaou, V.; Jones, G. R.; Engelis, N. G.; Discekici, E. H.; Fleischmann, C.; Willenbacher, J.; Hawker, C. J.; Haddleton, D. M., Universal Conditions for the Controlled Polymerization of Acrylates, Methacrylates, and Styrene via Cu(0)-RDRP. J. Am. Chem. Soc. 2017, 139 (2), 1003-1010.
21. Zhang, Q.; Wilson, P.; Li, Z.; McHale, R.; Godfrey, J.; Anastasaki, A.; Waldron, C.; Haddleton David, M., Aqueous Copper-Mediated Living Polymerization: Exploiting Rapid Disproportionation of CuBr with Me6TREN. J. Am. Chem. Soc. 2013, 135 (19), 7355-63.
22. Matyjaszewski, K.; Tsarevsky, N. V.; Braunecker, W. A.; Dong, H.; Huang, J.; Jakubowski, W.; Kwak, Y.; Nicolay, R.; Tang, W.; Yoon, J. A., Role of Cu0 in Controlled/\"Living\" Radical Polymerization. Macromolecules 2007, 40 (22), 7795-7806.
23. Rosen, B. M.; Percec, V., Single-Electron Transfer and Single-Electron Transfer Degenerative Chain Transfer Living Radical Polymerization. Chem. Rev. 2009, 109 (11), 5069-5119.
24. Konkolewicz, D.; Krys, P.; Matyjaszewski, K., Explaining Unexpected Data via Competitive Equilibria and Processes in Radical Reactions with Reversible Deactivation. Accounts of Chemical Research 2014, 47 (10), 3028-3036.
