Two-Faced Molecule Could Lead to Huntington’s Disease Treatment
Researchers from Carnegie Mellon University’s Institute for Biomolecular Design and Discovery (IBD) and Center for Nucleic Acids Science and Technology (CNAST), Danith Ly and postdoctoral associate Shivaji Thadke, have developed a two-faced synthetic nucleic acid that shows promise as a treatment for Huntington’s disease and other neurodegenerative and neuromuscular diseases caused by excessive repetition of nucleobase triplets in the genetic code.
Huntington’s disease is a genetic disorder that causes the progressive degeneration of nerve cells in the brain. Symptoms, which include changes in personality, mood and mobility and the ability to speak and swallow, normally appear between the ages of 30 and 50 and worsen over the course of 10 to 25 years. Huntington’s disease is fatal and there is no cure.
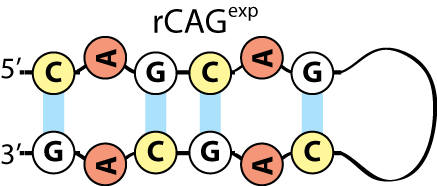
Huntington’s disease is the result of a mutation in the huntingtin gene that causes the expansion of the cytosine-adenine-guanine (CAG) triplet. In people without the mutation, the triplet will repeat approximately 35 times. In those with the mutation, the triplet repeats more than 50 times. When the gene is transcribed into RNA, the chain of repeats forms a hairpin loop that can trap proteins that play a vital role in protecting neurons and RNA splicing, among other mechanisms. The more repeats a person has, the longer the loop will be, allowing more proteins to be captured and increasing the severity of the disease.
In Huntington's disease, the repetition of the CAG triplet creates a hairpin loop that can trap proteins.
“Diseases like Huntington’s, ALS and muscular dystrophy, which have complicated, life-stealing symptoms, are caused by the repeat of only three nucleobases, which seems so simple,” said Ly, professor of chemistry in the Mellon College of Science. “If we can stop proteins from being sequestered in this hairpin, we believe we can help improve the symptoms of these diseases.”
Ly and Carnegie Mellon’s IBD and CNAST are leaders in the creation and development of peptide nucleic acids (PNAs). PNAs contain the same nucleobases as RNA and DNA and are synthetic analogs to the genetic molecules. PNAs can be programmed to correspond to genetic sequences that cause disease, allowing them to hunt down and bind with detrimental sequences to block the gene from malfunctioning.
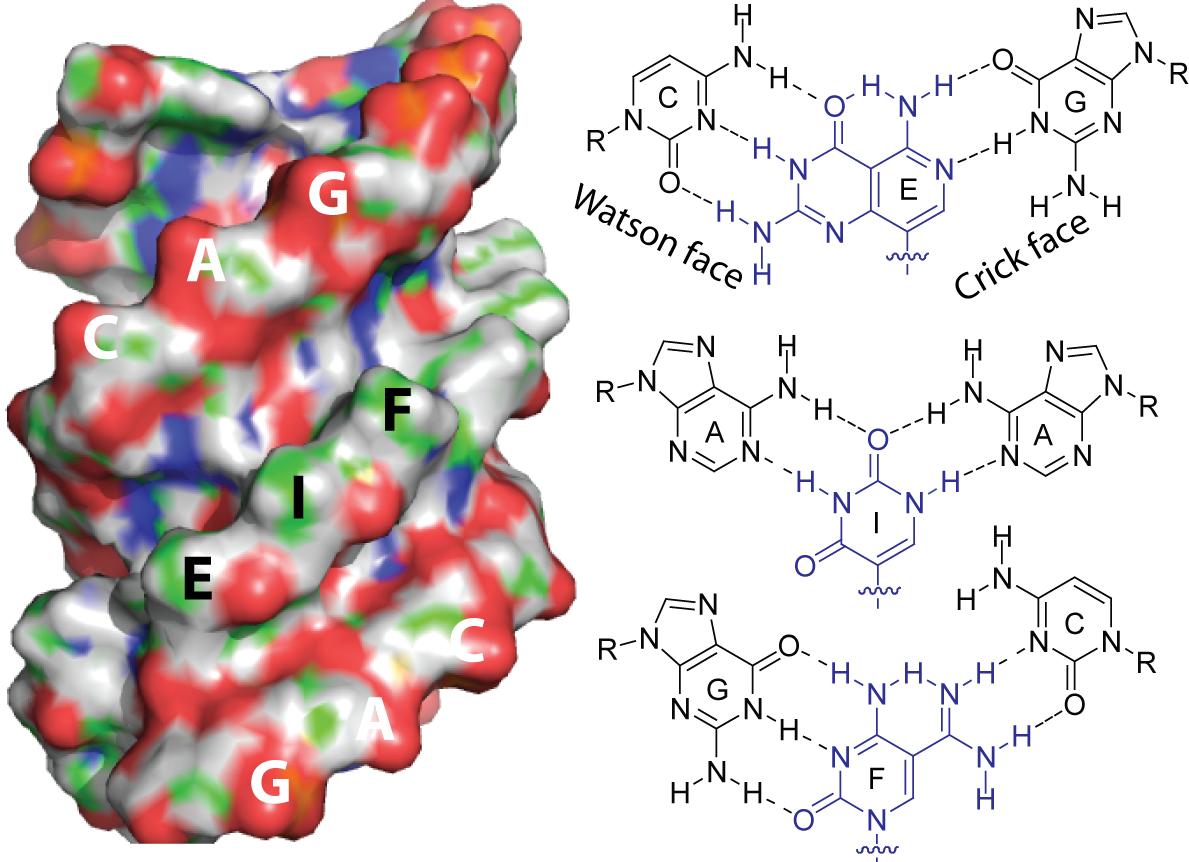
In 2014, CNAST received a $3.1 million gift from the DSF Charitable Foundation to develop the next generation of PNA technology. Ly turned his focus to developing Janus PNAs. Named after the two-faced Roman god, Janus PNAs are double-sided, allowing them to bind to both strands of a DNA and RNA molecule.
This hairpin structure in the Huntington’s RNA seemed that it would be a promising target for the Janus PNAs.
Ly created a 3 base-pair Janus PNA that corresponded with the repeated CAG triplet in Huntington’s disease. In the lab, they were able to demonstrate that the Janus PNA was able to invade the hairpin structure created by the repeating triplets and bind to each side. They believe that if enough Janus PNAs invade and bind to the hairpin structure, they can prevent neuroprotective proteins from binding and getting trapped in the hairpin. This would make the proteins available to shield the neurons and stave off the symptoms of Huntington’s disease.
The Janus PNA molecule can invade and bind to both sides of the hairpin loop. Researchers hope that this could prevent proteins from collecting within the loop.
“This wouldn’t have been possible without the DSF Charitable Foundation’s sustained support. They took a risk on an idea than many people said wasn’t possible,” said Ly. “While we still have a long way to go, we’ve shown that there is a possibility that this small, three-nucleotide molecule could lead to promising new treatments for devastating diseases, like Huntington’s disease.”
The research group published a proof-of-concept study in the journal Biochemistry that meticulously characterized their Janus PNA molecule. While there still is more research to be done, including testing the technique in cells and animal models, Ly believes that this could be a platform for treatments for the more than 20 diseases that are known to be caused by excessive repeats of nucleobase triplet.
CMU licensed the technology to a Pittsburgh-based startup company to start developing lead candidates for the treatment of neuromuscular and neurodegenerative disorders.
Originally published: https://www.cmu.edu/mcs/news-events/2018/0914_Janus-PNA_Huntingtons.html