Gold Nanoclusters Make Sharp Transition from Nonmetallic to Metallic
By Jocelyn Duffy
Media Inquiries- Mellon College of Science
It takes a mere 33 atoms for a gold nanoparticle to transition between its nonmetallic and metallic states according to new research published by Carnegie Mellon University chemists in the Journal of the American Chemical Society. Their experimental observations, which go against 50 years of theoretical predictions, reveal the critical size at which the metallic bond is formed. Their findings will help advance the design of functional nanomaterials.
The research was completed in the lab of Carnegie Mellon Chemistry Professor Rongchao Jin, a world leader in creating precisely-sized gold nanoparticles. Jin’s work has provided a greater understanding of the properties of these nanoclusters, which has in turn allowed them to be developed for use in a variety of fields, including manufacturing, imaging and biomedicine.
The metallic bond is among the most mysterious of the three types of chemical bonds. Large nanoclusters are known to be metallic and small nanoclusters are nonmetallic, but scientists didn’t know at what intermediate size the energy gap closes and the metallic state emerges.
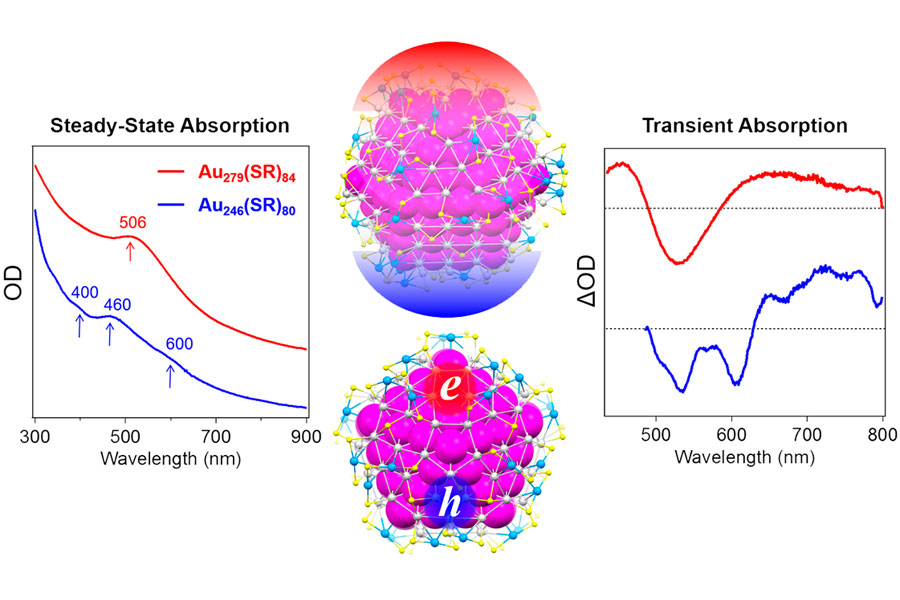
Using size-focusing and transformation techniques previously developed by Jin’s lab, Ph.D. student Tatsuya Higaki and postdoctoral researcher Meng Zhou created precisely-sized gold nanoclusters and excited them using femtosecond laser pulses. They observed the nanoclusters optical properties using ultrafast spectroscopy, which took timelapse snapshots of the electrons’ speedy movements. From the excited state dynamics, they were able to see if the particles exhibited metallic or nonmetallic behaviors.
They found that the transition between nonmetallic and metallic happened between gold clusters that contained 246 atoms and 279 atoms. The experimental results fly in the face of what the researchers had expected to see based on theoretical research. Studies dating back to the 1960s predicted that there would be a smooth transition between the nonmetallic and metallic states as the particles grew larger and larger and that the transition would be temperature dependent. The Carnegie Mellon team found neither to be true.
“A long standing, fundamental question in nanoscience is at what size the metallic state emerges,” said Jin. “It is surprising that a mere 33 gold atom difference causes the electronic-state transition.”
Gold nanoclusters have a wide variety of practical applications ranging from bio-imaging to catalysis. Metallic nanoclusters are of particular interest because they hold unique optical properties, knowing when the transition to the metallic state occurs will help accelerate the design of functional nanomaterials with tailored electronic and optical properties.
The findings also will help to advance theoretical studies of gold nanoclusters by giving researchers a more complete view of the electronic structures within the particles.
Additional study authors include Kelly J. Lambright and Kristin Kirschbaum of the University of Toledo Department of Chemistry and Biochemistry and Matthew Y. Sfeir of Brookhaven National Laboratory.
The research was funded by the Air Force Office of Scientific Research and used resources of the Center for Functional Nanomaterials, a U.S. Department of Energy Science Facility at Brookhaven National Laboratory.
Originally published: https://www.cmu.edu/mcs/news-events/2018/0515_nanoparticle-metallic-transition.html