CMU/NIST Partnership on Neutron Scattering in Life Sciences
CMU Physics Partners with NIST to Promote Neutron Scattering in the Life Sciences

NCNR Comprehensive Grant
The National Institute of Standards and Technology (NIST), an agency of the U.S. Department of Commerce, awarded a $2.5M Comprehensive Grant to CMU that will support a Center of Excellence Initiative to promote neutron scattering in the life sciences. The award makes CMU a key partner of the NIST Center for Neutron Research (NCNR) in neutron-based structural biology and aims to boost the application of neutron scattering techniques in the biological and biomedical sciences.
In this project, the CMU Physics Department, with its strong links to the Pittsburgh life science community that encompasses major national universities and the University of Pittsburgh School of Medicine (UPSM), aims to facilitate the translation of biological experimental systems to neutron measurements by lowering technological barriers for users whose professional and academic training is outside of physics. This will facilitate the characterization of high-resolution structures that help solve biologically important problems and thus raise the visibility of neutron scattering methods in the biological and biomedical community.
Beacon Science Projects within the Comprehensive Grant will pursue high-key biomedical research to demonstrate the power of neutron-based techniques in biology. In their work at the NCNR, CMU physics faculty Mathias Lösche and his group spearheaded the development of neutron-based studies of protein complexes with fluid phospholipid membranes, and the methodology they developed will be utilized in these Beacon Projects.
Beacon Science Project: KRas mediated cell signaling in cancer research
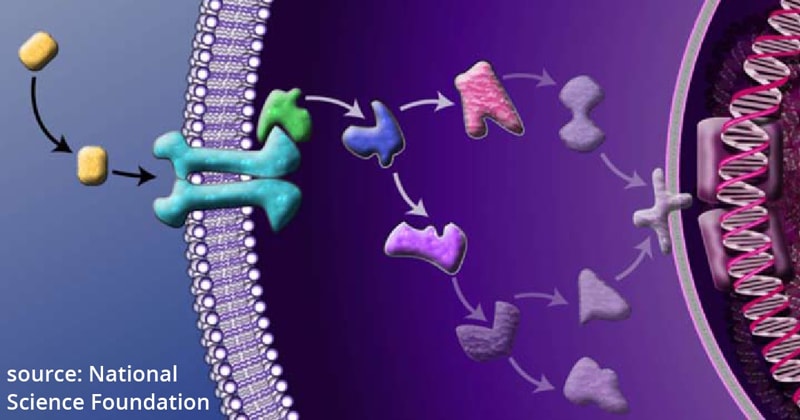
Cell signaling encompasses a host of processes that transmit chemical signals, such as hormonal stimuli, from the outside world into biological cells, eventually causing responses on the level of gene expression. Cascades of protein-protein interactions transmit these signals which often originate at the plasma membrane that forms the envelope of the cell.
Such processes are currently the object of intense studies in biomedical research because, when they fail, they can cause disease. Understanding these processes at the molecular level can also reveal a better understanding of how cells are organized.
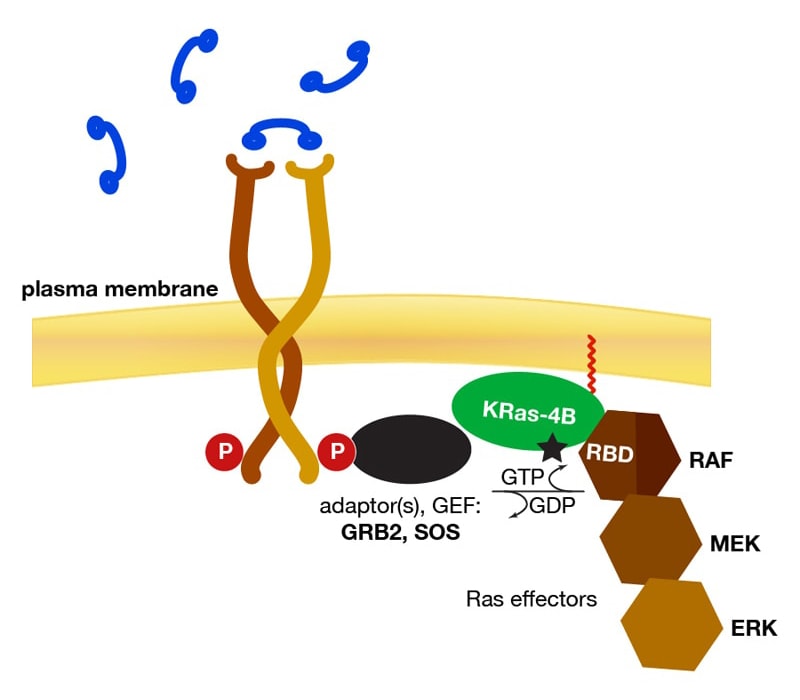
One particular signaling pathway involves the oncoprotein KRas. Deficiencies in this process notoriously lead to a family of tumors that remain untreatable because it is still unknown how Ras proteins activate the effectors that form its downstream targets.
The Lösche group at CMU Physics designed artificial membrane systems that are realistic physical models of biological membranes and optimized these systems for investigations with specular neutron reflectometry. NR is a mode of neutron scattering in which a highly collimated neutron beam is reflected from an atomically smooth carrier substrate that supports a single bilayer membrane. The group has also optimized data evaluation procedures and developed techniques to interpret NR results in the context of high-resolution protein structures from x-ray crystallography or NMR spectroscopy.
 Lösche group members involved in the project (left to right):
Lösche group members involved in the project (left to right):Frank Heinrich; PhD students Dennis Michalak, Chris Kervick, Rebecca Eells and Brad Treece; Mathias Lösche.
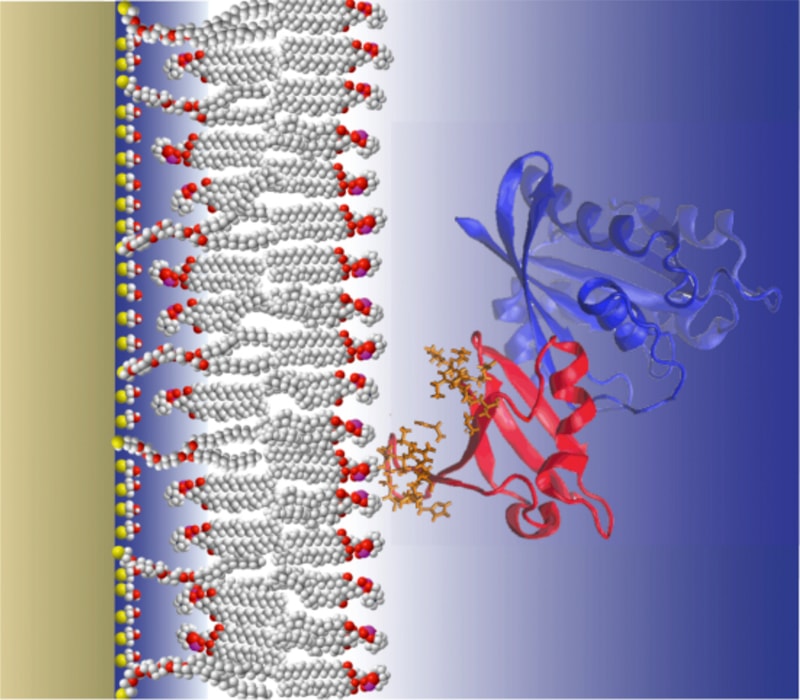
CMU Physics Associate Research Professor Frank Heinrich and coworkers in the CMU Physics Department now determined a draft structure of KRas with its downstream effector RAF-RBD. Together with Dr. A. Stephen and collaborators within the Ras Initiative located at the National Cancer Institute, they plan to investigate the details of KRas interactions with its related adaptor and effector proteins on the membrane in the scope of this project.