MCS Announces Recipients of DSF Rapid Seed Funding for COVID-19 Research
By Ben Panko
The Mellon College of Science (MCS) has awarded funding to five projects aimed at advancing diagnostics, treatments and vaccines for COVID-19. The grants are part of an innovative block grant program for interdisciplinary basic life science research supported by a generous $4 million gift from the DSF Charitable Foundation and administered by MCS.
This round of rapid seed funding was designed to help researchers establish a research program that could then seek other sources of funding. Projects from MCS and the College of Engineering were funded, and include the following:
Developing SARS-CoV-2 Antiviral Therapeutics with Artificial Intelligence Accelerated Molecular Modeling and Virtual Screening
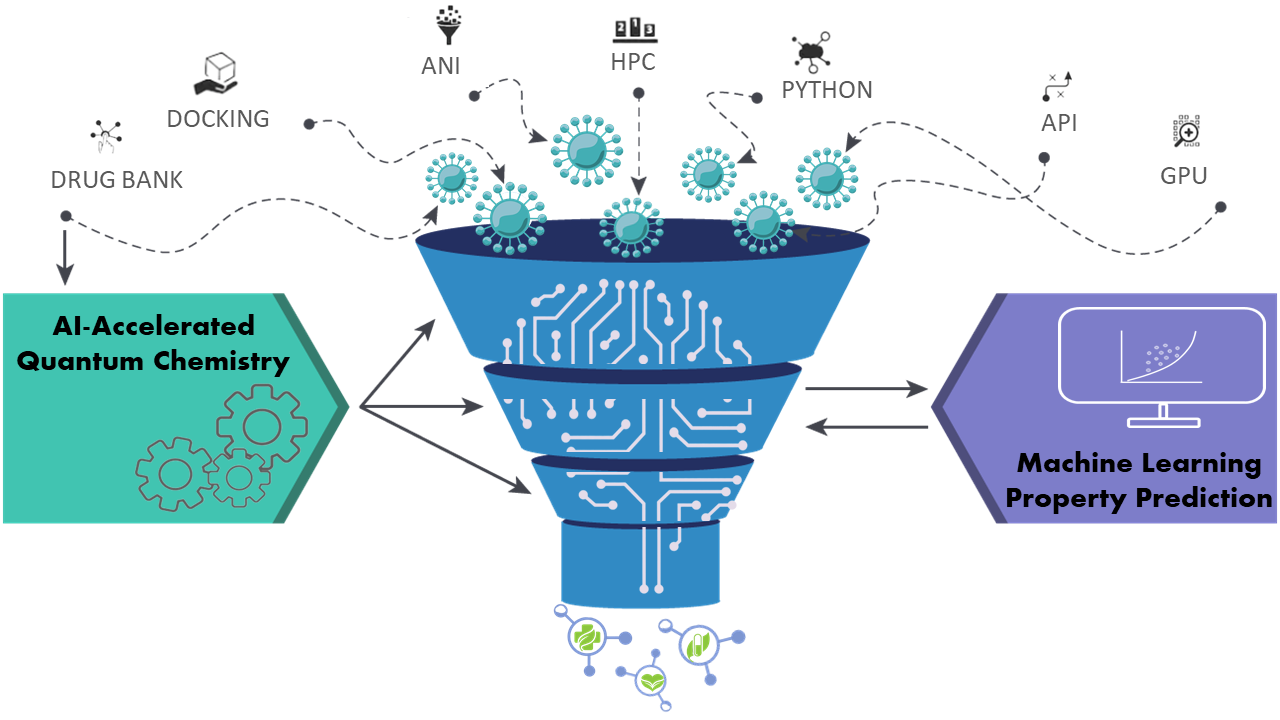
Associate Professor of Chemistry Maria Kurnikova and Assistant Professor of Chemistry Olexandr Isayev will apply machine learning, artificial intelligence and structural molecular modeling techniques to aid the design of new drugs and the repurposing of existing ones for treating the SARS-CoV-2 virus. Using the lab's quantum mechanical methods that are accelerated by AI, a large library of purchasable molecules will be parameterized and then run through a structure-based virtual screening. Then molecular dynamics and free energy simulations will provide data for deep learning algorithms that could identify the best candidates for testing in partner labs. Through this more efficient and accurate method of drug discovery, the researchers aim to find an antiviral drug that could bind to the viral protein of SARS-CoV-2 and inhibit enzyme activity.
Evaluation of Hybrid Microneedle Arrays for Effective COVID-19 Vaccination
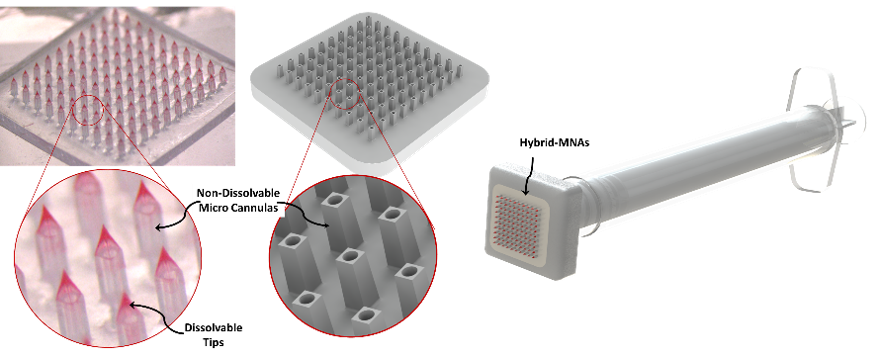
Burak Ozdoganlar, Ver Planck Professor of Mechanical Engineering and Biomedical Engineering, and Phil Campbell, research professor of biomedical engineering and the Engineering Research Accelerator, are working to prove the viability of a new vaccine delivery method, hybrid microneedle arrays, for combating COVID-19 and future pandemics. Microneedle arrays contain hundreds of miniscule needles arranged in a patch that can be used to painlessly and effectively deliver vaccines. However, these standard arrays are not the most effective at delivering different formulations of vaccines. In Ozdoganlar and Campbell's hybrid microneedle arrays, hundreds of cannulas are capped with dissolvable polymers that can precisely and quickly infuse vaccines into the skin. With their funding, the pair plan to test the hybrid microneedle arrays on rodent models and human skin explants.
Rapid Detection of COVID-19 Antibodies Using Enhanced Electrochemical Reaction via 3D-Printed Micropillar-Based Lab-on-a-Chip Biosensor
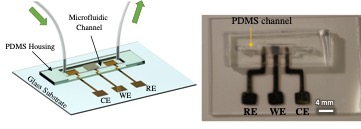
Using 3D-printed electrochemical sensors that were developed at Carnegie Mellon University, the test will be able to detect COVID-19 antibodies on the third to fifth day after viral infection in one minute with an accuracy of 80% to 90%. The testing platform will consist of gold micropillar array electrodes adorned with reduced graphene oxide and functionalized with recombinant viral antigens that will be integrated with a microfluidic device. The results from the platform will be able to be read on a smartphone. Panat is developing the test with serum samples from the University of Pittsburgh Medical Center.
Developing a Robust Diagnostic for COVID-19 Immunity
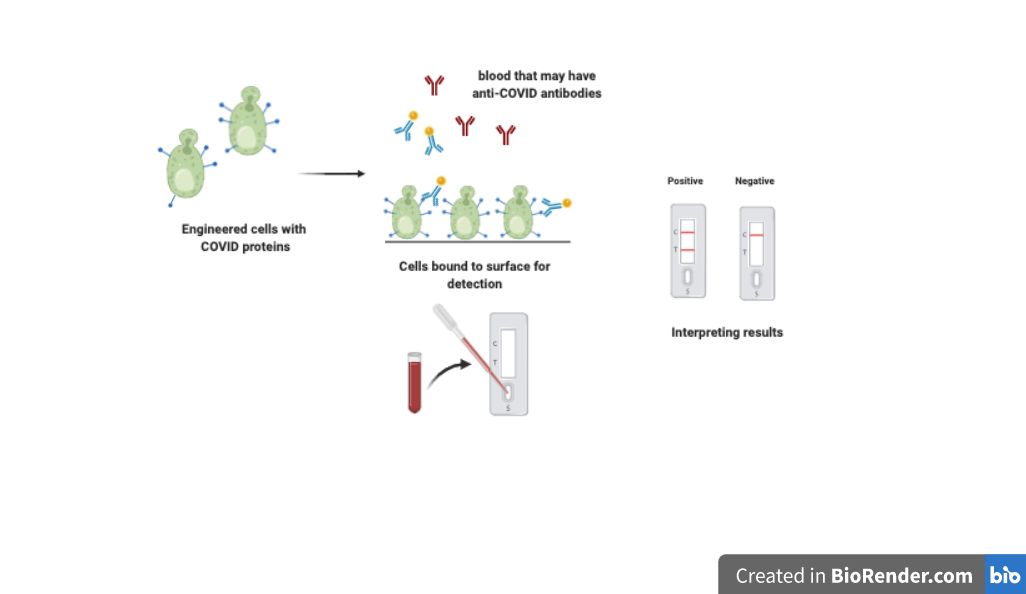
Anne Skaja Robinson, the Trustee Professor and department head of chemical engineering, will develop an inexpensive test to detect neutralizing antibodies for COVID-19 in a person's serum. The test will use a combination of cellular reporters and lateral assay flows, similar to pregnancy tests, to enhance its sensitivity, stability and shelf life.
Fragment-Based Drug Discovery of SARS COV-2 Protease Inhibitors

Professor of Biological Sciences Gordon S. Rule will work to develop new potential lead compounds for COVID-19 therapeutics using fragments that bind to viral enzymes. Typical drug discovery efforts screen large libraries of compounds for potential enzyme inhibitors, but this work often only produces a few viable candidates; and those compounds can be difficult to optimize for therapeutic use. Rule's lab will identify small organic fragments that bind to a viral enzyme, and then synthetically link the fragments together to create robust new compounds for potential drugs. The group will focus on two enzymes in the SARS-CoV-2 virus that have no homologs in humans so any inhibitors identified will not interfere with normal processes in the body.