Pittsburgh Cold Study 3
Study Description
Pittsburgh Cold Study 3 (PCS3) was a prospective viral challenge study with data collected from 2007-2011 among healthy volunteers ages 18-55 (mean 30.1; SD 10.9). This study extended work on the role of childhood environment in common cold susceptibility by including additional retrospective measures of childhood and adolescent experience, such as parental social participation, parental bonding, family structure and relationships, neighborhood physical and social environments, and childhood physical health. Numerous other social, psychological and behavioral measures were administered during the pre-challenge baseline period as well, including assessments of current social relationships, personal attributes, stressful life events, personality characteristics, and health practices. PCS3 also included detailed daily interviews (14 days) with participants to assess health behaviors, mood, and daily social interactions. One of the novel features of PCS3 is that it introduced several additional biological assessments prior to viral challenge, including markers of biological aging (e.g., telomere length in lymphocytes, oxidative stress), cardiovascular and cortisol reactivity to acute laboratory stress, and cytokine and glucocorticoid and adrenergic receptor genotypes. Post-challenge measures, in addition to standard virology, included local (nasal secretions) cytokines (interleukin [IL]-1β, IL-6, IL-8, IL-10, IFN-α, and TNF-α).
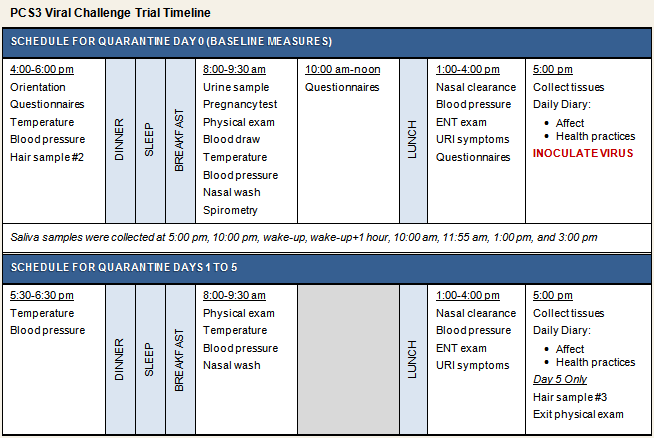
Participants were 123 men and 90 women from the Pittsburgh, Pennsylvania metropolitan area who responded to newspaper advertisements and were judged to be in good health after a medical examination. Prior to enrollment, volunteers completed a telephone screening interview followed by an in-person physical health evaluation conducted by a study physician. To maximize the rate of infection, only eligible volunteers with viral-specific antibody titers ≤4 were included in the study (see Human Subjects for information on additional inclusion and exclusion criteria). After completing baseline psychosocial questionnaires and biological assessments (e.g., biological aging markers, saliva cortisol), participants were administered nasal drops containing rhinovirus 39 (RV39). They were then followed in quarantine for 5 days and monitored for development of infection and objective signs of illness (see viral challenge timeline below). Approximately 28 days after virus exposure, blood was collected for serological testing. Participants were considered to have a cold if they were both infected with the challenge virus and met illness criteria. All individuals who completed the study received $1,000 for their participation, plus an additional $60 if they provided hair samples for cortisol analysis.