Infection, Upper Respiratory Signs and Symptoms, and Clinical Colds
The primary outcome for each of the 5 studies comprising the Common Cold Project was the development of a clinical cold following experimental exposure to a virus that causes upper respiratory illness. In the present context, a clinical cold is defined as the condition of both having been infected with the challenge virus and expressing criterion signs and/or symptoms suggestive of upper respiratory tract involvement. Throughout this documentation, the terms “clinical cold”, “clinical illness”, and simply “cold” are used interchangeably to refer to this condition. Detailed information on the criteria used to define infection and suggestive upper respiratory pathology, as well as descriptions of the specific measures used to obtain virological and other cold-related data can be obtained by clicking on the relevant link in the table below.| ANALYSIS NOTE: |
|
The 4 studies conducted in Pittsburgh (PCS1, PCS2, PMBC, PCS3) all determined the presence of criterion signs and symptoms of illness using the same 2 assessment techniques, with one being based on objective disease markers (signs) and the other on a standardized set of self-reported symptoms. By comparison, the BCS assessed criterion signs and symptoms based on physician diagnosis. Thus, while data from the 4 Pittsburgh studies can be aggregated without concern about interstudy differences in how these outcomes were measured, inclusion of BCS data would require the assumption that differing techniques for assessing illness outcomes are comparable in regard to their capacity to identify the presence of a clinical cold. |
| Category | Specific Measure | BCS |
PCS1 | PCS2 | PMBC | PCS3 |
| Definitions of Major Outcomes | ||||||
| Infection | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Objective clinical illness (cold) criteria | ✔ | ✔ | ✔ | ✔ | ||
| Physician diagnosis-based clinical illness (cold) criteria | ✔ | |||||
| Subjective clinical illness (cold) criteria | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Virology | ||||||
| serum | Baseline & post-challenge virus-specific Ab | ✔ | ✔ | ✔ | ✔ | ✔ |
| serum | Baseline total (nonspecific) Ab | ✔ | ||||
|
nasal secretions |
Isolation & confirmation of challenge virus | ✔ | ✔ | ✔ | ✔ | ✔ |
|
nasal secretions |
Quantification of challenge virus | ✔ | ✔ | ✔ | ||
|
nasal secretions |
Baseline local total (nonspecific) Ab | ✔ | ||||
|
nasal secretions |
Baseline & post-challenge local virus-specific Ab | ✔ | ||||
| Objective Cold Signs | ||||||
| quantitative | Nasal mucociliary clearance function | ✔ | ✔ | ✔ | ✔ | |
| quantitative | Nasal mucus weights | ✔ | ✔ | ✔ | ✔ | ✔ |
| quantitative | Tissue counts | ✔ | ||||
| observed | Clinician observation records | ✔ | ||||
| Subjective Cold Symptoms | ||||||
| Self-report (questionnaire) | ✔ | ✔ | ✔ | ✔ | ||
| Self-report (clinician interview) | ✔ | |||||
Infection
A necessary precondition for identifying participants who developed clinical upper respiratory illness was verified infection with the challenge virus. To determine whether participants had become infected subsequent to challenge, all 5 studies employed criteria described by Gwaltney and colleagues (1989) that involved evidence of viral replication (shedding) and/or changes in serum specific antibody titer. Specifically, participants were considered to be infected with the challenge virus if either of the following two conditions had been met:
- Recovery of the challenge virus in nasal secretions on any of the post-challenge days
- A 4-fold or greater increase in serum virus-specific antibody titer between the pre-viral challenge baseline and 28 days post-challenge
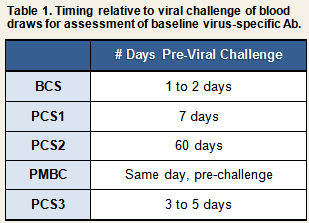
In BCS, a 4-fold or greater increase in viral-specific antibody titer was used as a criterion to determine infection only among participants who had been exposed to one of the three rhinoviruses that were used in that study (RV2, RV9, and RV14). Because neutralizing tests were not available for respiratory syncytial virus (RSV) and coronavirus (CV), ELISAs were used to assay for specific antibody in the serum and nasal secretions of participants exposed to either of these two viruses. Based on these data, infection with RSV and CV was determined based on the recovery of challenge virus in nasal secretions or on a >2 standard deviation increase in serum specific immunoglobulin A (IgA) or IgG relative to the mean specific IgA or IgG value for unchallenged controls.
Upper Respiratory Tract Involvement
The 4 Pittsburgh studies have used two sets of criteria to determine the presence of upper respiratory tract involvement, with the first being based on participants’ self-reports of perceived physical symptoms and the second on objectively assessed signs of upper respiratory pathology. Henceforth, we will refer to these as the subjective definition (or subjective criteria) and the objective definition (or objective criteria), respectively. The subjective criteria have been used widely in the virology literature. We prefer the objective criteria because they focus on observable disease pathology and thus completely eliminate any possibility that the outcome reflects biases in how individuals perceive or report their bodily sensations.
The criteria for determining upper respiratory involvement used in the BCS are based on a combination of objective and subjective information. As described in the following section, determinations of sign and symptom criteria were based on the presence of symptoms (assessed by a combination of participants’ responses to clinician interviews and clinicians’ own observations during physical exam; see Clinical Interview and Observations), and the clinician’s judgment of sign/symptom severity based on his interpretation of interview and observational data.
Again, we emphasize that satisfaction of any of the several sets of sign and symptom criteria discussed below is suggestive of clinical illness only in the context of a verified infection. We also reiterate that one must take into account the differences between the BCS and the 4 Pittsburgh studies in how colds are defined when incorporating BCS data into an aggregated data set.
Physician Diagnosis (BCS)
Participants were examined daily by the study clinician for three days before and six days after viral challenge, and a daily record was made of the presence and severity of signs and symptoms associated with a clinical cold (See Table 2).
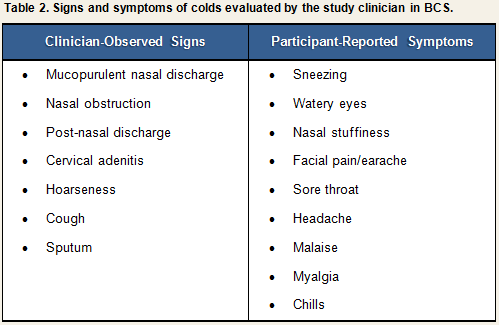
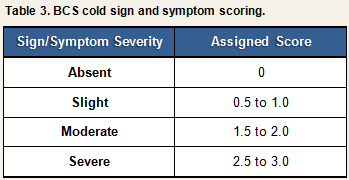
Each sign or symptom was rated by the clinician as being absent, slight, moderate, or severe and assigned a numeric score based on the algorithm displayed in Table 3. Scores for individual signs/symptoms were summed within each day to create daily total sign/symptom scores.
At the end of the trial (Quarantine Day 6), the clinician judged the severity of each participant’s cold on a scale ranging from 0 (no cold) to (4) (severe cold). Participants infected with the challenge virus were determined to have developed a clinical cold if they received a clinician rating of 2 (mild cold) or higher, and had a symptom score greater than 0 during the 5 days that were used to calculate symptoms (Quarantine Days 2-6). Figure 1 provides a visual representation of the decision paths used to determine whether participants developed a clinical illness using the physician diagnosis criteria.
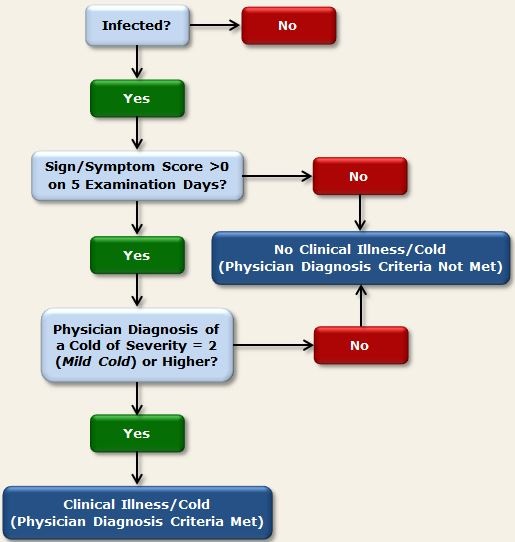
Figure 1. Physician diagnosis criteria used in BCS to determine clinical illness
<< Download PowerPoint Slide >>
Example using physician diagnosis criteria:
Cohen, S., Tyrrell, D. A. J., & Smith, A. P. (1991). Psychological stress and susceptibility to the common cold. New England Journal of Medicine, 325, 606-612.
Criteria for Objective Signs of Upper Respiratory Pathology (PCS1, PCS2, PMBC, PCS3)
Using the objective criteria (Cohen et al., 1997), participants were determined to have developed a clinical cold if they were both infected with the challenge virus and met either of the following criteria:
- Total baseline-adjusted mucus weight (i.e., summed across all post-challenge days) of 10g or more
- Average (across all post-challenge days) baseline-adjusted nasal mucociliary clearance time of 7 minutes or longer (or total clearance time across 5 post-challenge days of 35 minutes or longer, as reported in some publications).
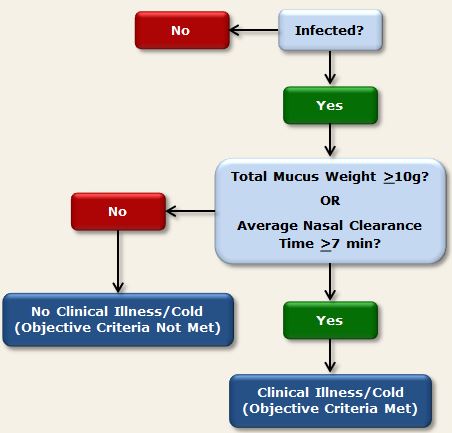
Figure 2. Objective criteria used in PCS1, PCS2, PMBC, and PCS3 to determine clinical illness
<< Download PowerPoint Slide >>
Example using objective criteria:
Cohen, S., Doyle, W. J., Skoner, D. P., Rabin, B. S., & Gwaltney, J. M., Jr. (1997). Social ties and susceptibility to the common cold. Journal of the American Medical Association, 277, 1940-1944.
Criteria for Subjective Upper Respiratory Symptoms (PCS1, PCS2, PMBC, PCS3)
We have favored the objective criteria for upper respiratory involvement described above because subjective assessments of symptoms and illness may be influenced by psychological variables. Such effects may occur independent of any influence of the objective signs of disease. However, historically, virologists studying risk factors for colds have used subjective criteria. Here we describe the modified Jackson Criteria (Gwaltney, Moskalski, & Hendley, 1980), which take into account individuals’ subjective ratings of 8 respiratory symptoms identified by Jackson and colleagues (1958) as being characteristic of the common cold. Importantly, satisfaction of subjective upper respiratory symptom criteria is suggestive of clinical illness only in the context of a verified infection.
Daily symptom reports
At the end of each day in quarantine, participants used a scale ranging from 0 (none) to 4 (very severe) to rate their experience during the past 24 hours of each of the following 8 symptoms of upper respiratory illness (see Daily Symptoms):
- nasal congestion
- runny nose
- sneezing
- cough
- sore throat
- malaise (feeling “under the weather”)
- headache
- chills
In addition to rating the severity of these 8 symptoms, participants were asked each day whether they thought that had a cold or flu.
Jackson Symptom Scores
Symptom severity ratings were summed within each day to create a daily Jackson Symptom Score (possible range, 0 to 32). Adjusted Daily Jackson Symptom Scores were computed by subtracting the DAY 0 (pre-challenge) Jackson Symptom Score from the Jackson Score reported on each subsequent post-challenge day. Adjusted scores were then averaged across all post-challenge days to create a Total Adjusted Jackson Symptom Score.
Modified Jackson Criteria
In the 4 cold studies that collected subjective symptom data, the Modified Jackson Criteria were used to determine whether participants’ subjective assessments of their cold symptoms were consistent with a diagnosis of clinical upper respiratory illness. These criteria are defined as
- having a Total Adjusted Jackson Symptom Score of 6 or more AND
- reporting having a cold or flu on any post-challenge quarantine day OR reporting rhinorrhea on 3 or more post-challenge days.1
Participants who both became infected with the challenge virus and whose symptom reports met the above-described criteria, were determined to have developed a cold by the subjective definition.2
Figure 3 below provides a visual representation of the decision paths used to determine the presence of clinical illness using the subjective criteria.
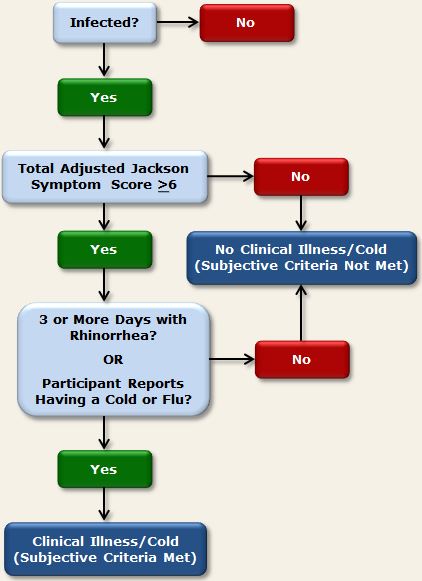
Figure 3. Subjective criteria used in PCS1, PCS2, PMBC, and PCS3 to determine clinical illness
<< Download PowerPoint Slide >>
Example of paper using subjective criteria:
Cohen, S., Doyle, W. J., Turner, R. B., Alper, C. M., & Skoner, D. P. (2003). Emotional style and susceptibility to the common cold. Psychosomatic Medicine, 65, 652-657.
| ANALYSIS NOTE: |
|
BCS also collected information on symptoms, but they were not collected for purpose of analysis, but rather for use by the clinician in making a clinical judgment. In this study the data were obtained through a combination of participant responses to clinician interviews and clinician observation records (see Clinical Interview and Observations). Accordingly, while the BCS symptom data provide an indication of illness severity, they do not provide a pure measure of participants’ own subjective assessments of their symptoms, and thus are neither comparable to the symptom data collected in the other 4 studies nor amenable to the modified Jackson Criteria described below. |
Notes
1 Symptom ratings used in the creation of the original Jackson criteria (Jackson et al., 1958) were rated on a scale of 0 to 3, and the criterion score was a total of 14 points over a post-challenge observation period of 6 days. As with the modified criteria, individuals also had to report having a cold or experiencing 3 or more days with rhinorrhea.
2 It should be noted here that the symptoms comprising the Jackson Symptom Score are characteristic of the common cold. In the PMBC study, participants in the first 5 trials were challenged with influenza A (n=38) whereas those in the remaining 6 trials were challenged with a common cold virus (rhinovirus [RV] 39; n=155). Though both viruses produce upper respiratory illness, the 8 Jackson Symptoms do not include additional physical complaints that accompany the flu (i.e., fever, sweating, and muscle and joint aches). Accordingly, criteria appearing in papers published using PMBC data may differ somewhat from the Modified Jackson Criteria.
References
Gwaltney, J. M. Jr., Colonno, R. J., Hamparian, V. V., & Turner, R. B. (1989). Rhinovirus. In N. J. Schmidt & R. W. Emmons (Eds). Diagnostic procedures for viral, rickettsial, and chlamydial infections, 6th ed (pp. 579-614). Washington, D.C.: American Public Health Association.
Gwaltney, J. M. Jr., Moskalski, P. B., & Hendley, J. O. (1980). Interruption of experimental rhinovirus transmission. Journal of Infectious Diseases, 142, 811-815.
Jackson, G. G., Dowling, H. F., Spiesman, I. G., & Boand, A. V. (1958). Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity. Archives of Internal Medicine, 101, 267-278.