Assessment of Infection and Colds (BCS)
Table 1 below lists the virological measures and assessments of cold signs and symptoms that were used in the BCS to determine infection with the challenge virus and subsequent diagnosis of clinical upper respiratory illness. The criteria used for identifying infection and illness are described in detail below.
Table 1. Measures Used to Determine Infection and Clinical Illness in the BCS
| Category | Specific Measure |
| Virology | |
| serum | Baseline & Post-Challenge Virus-Specific Ab |
| serum | Baseline Total (nonspecific) Ab |
|
nasal secretions |
Isolation and Confirmation of Challenge Virus |
|
nasal secretions |
Baseline Local Total (nonspecific) Ab |
|
nasal secretions |
Baseline & Post-Challenge Local Virus-Specific Ab |
| Objective Cold Signs | |
| quantitative | Nasal Mucus Weights |
| quantitative |
Tissue Counts |
| observed | Clinician Diagnosis Based on Physical Exam |
| observed | Clinician Diagnosis Based on Participant Report |
Infection
A necessary precondition for identifying participants who developed clinical upper respiratory illness was verified infection with the challenge virus. To determine whether participants had become infected subsequent to challenge, the BCS employed criteria described by Gwaltney and colleagues (1989) that involved evidence of viral replication (shedding) and/or changes in serum specific antibody titer. Specifically, participants were considered to be infected with the challenge virus if either of the following two conditions had been met:- Recovery of the challenge virus in nasal secretions on any of the post-challenge days
- A 4-fold or greater increase in the virus-specific antibody titer between the pre-viral challenge baseline (1 to 2 days before viral challenge) and 28 days post-challenge
Upper Respiratory Tract Involvement
The criteria for determining upper respiratory involvement used in the BCS are based on a combination of objective and subjective information: (1) participants’ responses to clinician interviews in combination with clinicians’ own observations during physical exam (see Clinical Interview and Observations); and (2) the clinician’s judgment of sign/symptom severity based on his interpretation of interview and observational data. Again, we emphasize that satisfaction of these criteria is suggestive of clinical illness only in the context of a verified infection.Participants were examined daily by the study clinician for three days before and six days after viral challenge, and a daily record was made of the presence and severity of signs and symptoms associated with a clinical cold (See Table 2).
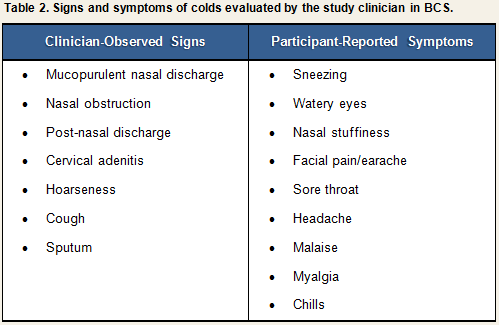
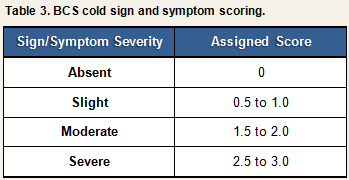
Each sign or symptom was rated by the clinician as being absent, slight, moderate, or severe and assigned a numeric score based on the algorithm displayed in Table 3. Scores for individual signs/symptoms were summed within each day to create daily total sign/symptom scores.
At the end of the trial (Quarantine Day 6), the clinician judged the severity of each participant’s cold on a scale ranging from 0 (no cold) to (4) (severe cold). Participants infected with the challenge virus were determined to have developed a clinical cold if they received a clinician rating of 2 (mild cold) or higher, and had a symptom score greater than 0 during the 5 days that were used to calculate symptoms (Quarantine Days 2-6). Figure 1 provides a visual representation of the decision paths used to determine whether participants developed a clinical illness using the physician diagnosis criteria.
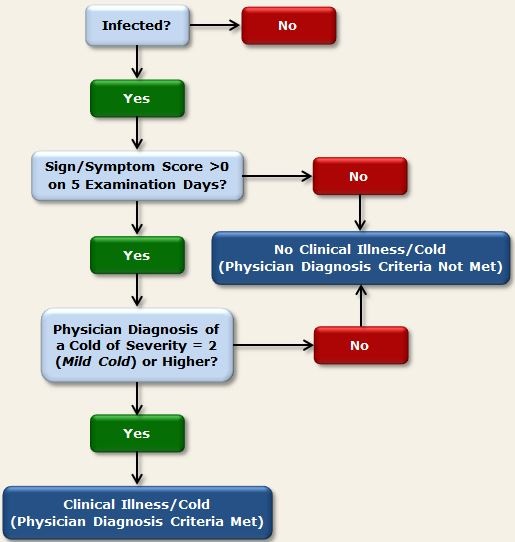
Figure 1. Physician diagnosis criteria used in BCS to determine clinical illness
References
Cohen, S., Tyrrell, D. A. J., & Smith, A. P. (1991). Psychological stress and susceptibility to the common cold. New England Journal of Medicine, 325, 606-612.Gwaltney, J. M. Jr., Colonno, R. J., Hamparian, V. V., & Turner, R. B. (1989). Rhinovirus. In N. J. Schmidt & R. W. Emmons (Eds). Diagnostic procedures for viral, rickettsial, and chlamydial infections, 6th ed (pp. 579-614). Washington, D.C.: American Public Health Association.