Single IRB (sIRB) for New Cooperative Research
Federal regulations for Human Subjects Research are summarized below: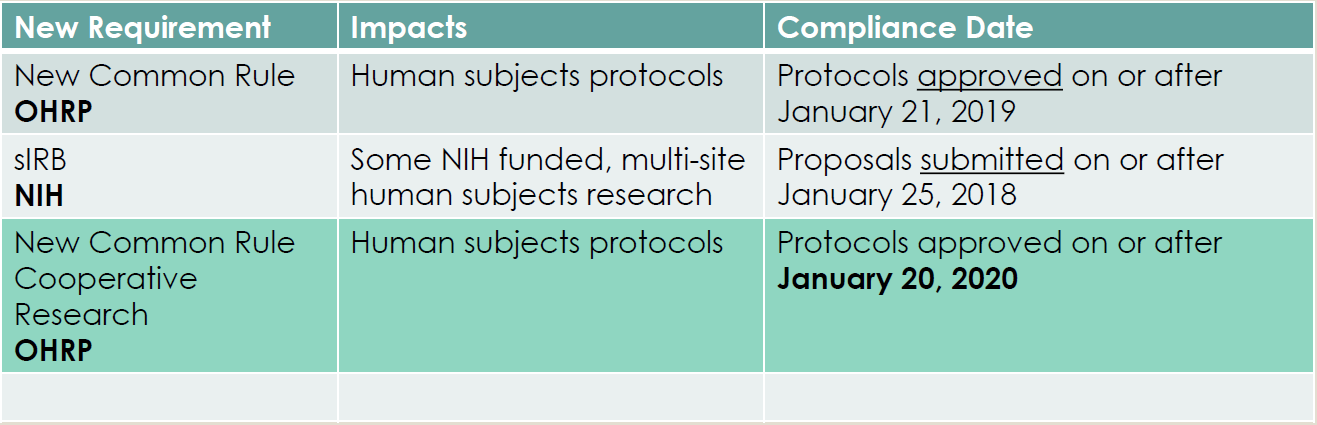
Federal regulations for Cooperative Research went into effect on January 20, 2020, and apply to federally funded Human Subjects Research (HSR)
- Protocols, involving cooperative research, approved on or after January 20, 2020 must follow the new Common Rule Cooperative Research Regulations.
- Protocols, involving cooperative research, approved prior to January 20, 2020 will continue under the current Common Rule
For federally funded HSR, Cooperative Research is defined as projects that involve more than one institution. Each institution is responsible for safeguarding the rights and welfare of human subjects at their institution.
Any institution located in the United States that is engaged in cooperative research must rely upon approval by a single IRB for that portion of the research that is conducted in the United States.
The reviewing IRB (or sIRB) will be identified by the Federal department or agency supporting or conducting the research or proposed by the lead institution subject to the acceptance of the Federal department or agency supporting the research.
Exceptions:
When more than sIRB is required by law ((including tribal law passed by the official governing body of an American Indian or Alaska Native tribe).
Research for which any Federal department or agency supporting or conducting the research determines and documents that the use of a single IRB is not appropriate for the particular context.
Contact: sirb@andrew.cmu.edu