Carnegie Mellon Researchers Create Biohybrid Nanoparticles That Could Revolutionize Drug Delivery
By Ben Panko
An international team of scientists led by Carnegie Mellon University researchers has engineered novel hybrid exosomes with polymer surfaces that can be used to precisely and consistently deliver therapeutics. The research has been published in the “Proceedings of the National Academy of Sciences.”
The genesis of this research came over drinks several years ago, according to Sushil Lathwal, a postdoctoral researcher at MIT, who received his Ph.D. in chemistry from Mellon College of Science in 2020, and Saigopalakrishna “Sai” Yerneni, a biomedical engineering doctoral student in the College of Engineering.
Yerneni had been working on understanding cell-to-cell communication and developing synthetic exosomes — miniscule extracellular vesicles that can transport a variety of cargo from cell-to-cell like mail carriers.
“What is unique about these ‘mail carriers’ is that they are optimized to travel in the body without attracting undue attention from the immune system, making them an ideal drug delivery vehicle,” Yerneni said.
In recent years, scientists have been working to harness these natural nanoparticles to deliver therapeutics, such as cancer drugs. However, natural exosomes are very unstable in storage and when circulating in the body, limiting their potential effectiveness for medical uses.
That’s where Lathwal came in.
"As we were watching the bubbles in our beer that resembled exosomes that night, we thought ‘could we combine polymer chemistry with exosomes to create a unique class of exosome-polymer hybrids for drug delivery applications?"
Sushil Lathwal
In his research, Lathwal had been working on automating the synthesis of polymer biohybrids using atom transfer radical polymerization (ATRP), the groundbreaking and robust method of controlled polymerization developed by CMU’s J.C. Warner University Professor of Natural Sciences Krzysztof Matyjaszewski in 1994.
“As we were watching the bubbles in our beer that resembled exosomes that night, we thought ‘could we combine polymer chemistry with exosomes to create a unique class of exosome-polymer hybrids for drug delivery applications?’” Lathwal said. That idea sparked a collaboration that came to involve researchers from Mellon College of Science, the College of Engineering and institutions in three other countries.
The 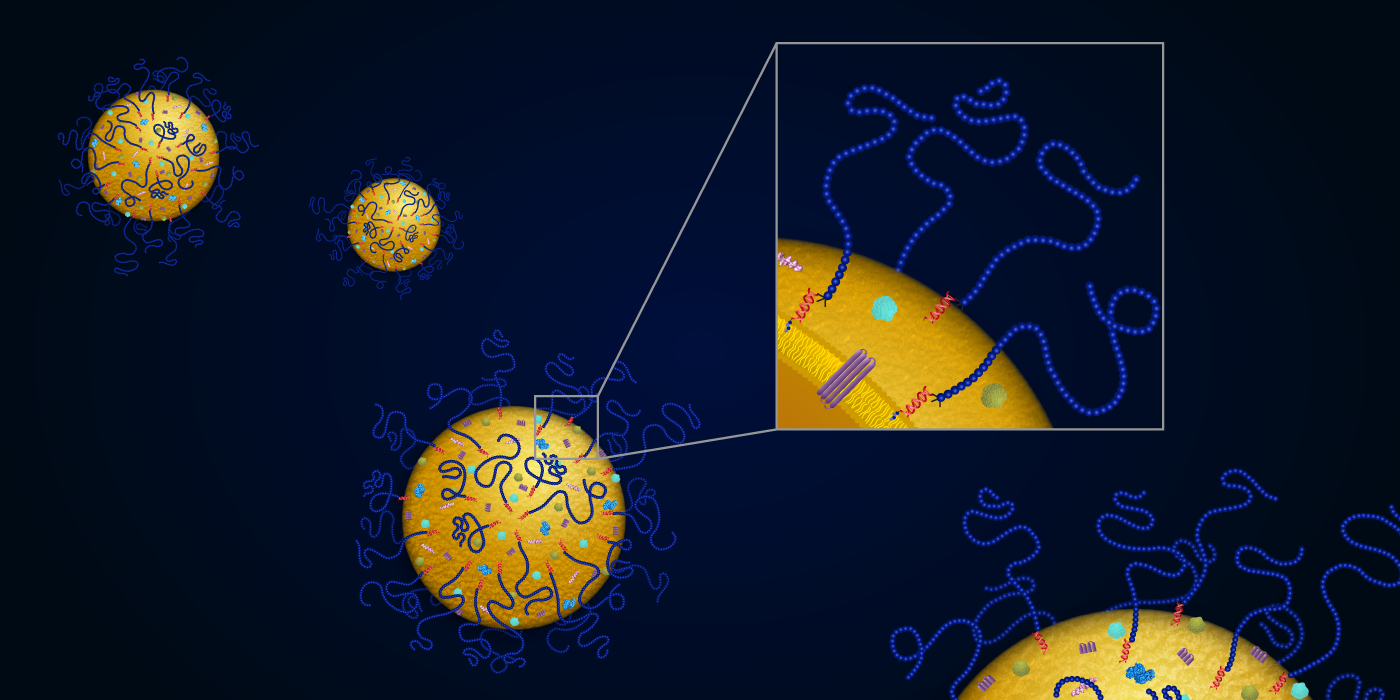 latest research from this scientific team builds on work published last year in the journal “ACS Nano,” which demonstrated the creation of an all-purpose platform for easily producing synthetic exosomes with a DNA-cholesterol tether that could be used to carry a wide variety of cargo to cells. In their study, the researchers harnessed the power of ATRP to create exosomes with thousands of polymer chains on their surfaces, known as exosome polymer hybrids. These biocompatible surface polymers serve as a protective shell for the exosome, making them more stable.
latest research from this scientific team builds on work published last year in the journal “ACS Nano,” which demonstrated the creation of an all-purpose platform for easily producing synthetic exosomes with a DNA-cholesterol tether that could be used to carry a wide variety of cargo to cells. In their study, the researchers harnessed the power of ATRP to create exosomes with thousands of polymer chains on their surfaces, known as exosome polymer hybrids. These biocompatible surface polymers serve as a protective shell for the exosome, making them more stable.
“We were able to improve the shelf life of exosomes from weeks to months,” Yerneni said. “But more importantly, we increased their circulation time in the blood by severalfold to provide them enough time to reach the target tissue.” While native exosomes are cleared from blood circulation in three hours, exosome-polymer hybrids remain in circulation for 12 hours post-injection.
“Researchers still need to work out how exosomes are synthesized by cells and why they are so effective at infiltrating cells and evading immune detection.”
Saigopalakrishna “Sai” Yerneni, biomedical engineering doctoral student, College of Engineering
“Researchers still need to work out how exosomes are synthesized by cells and why they are so effective at infiltrating cells and evading immune detection,” Yerneni said. “Only after they answer these basic questions will this new mode of drug delivery be ready for clinical application.”
Other authors on the paper include Matyjaszewski, Subha R. Das, associate professor of chemistry; and Phil G. Campbell, research professor of biomedical engineering and member of the Engineering Research Accelerator; Susanne Boye, Albena Lederer and Upenyu L. Muza from the Leibniz Institute of Polymer Research Dresden and Shuntaro Takahashi; and Naoki Sugimoto from the Frontier Institute for Biomolecular Engineering Research.
Funding for this study was provided by the National Science Foundation (DMR 1501324), CMU-Biohybrid Organ Center Seed Funding, the Ministry of Education, Culture, Sports, Science and Technology and the Japan Society for the Promotion of Science (No. JP17H06351).

