Bacterial Strains “Talk” to Each Other to Control Disease
Many bacteria communicate with each other through specialized signaling mechanisms, which are important to sense and respond to changes in the environment and in the human host. The Hiller lab studies the human pathogen, Streptococcus pneumonia (pneumococcus). In pneumococcus, these signals regulate gene expression and in doing so dictate whether pneumococcus asymptomatically colonizes the throat or disseminates thought out human tissues, leading to disease.
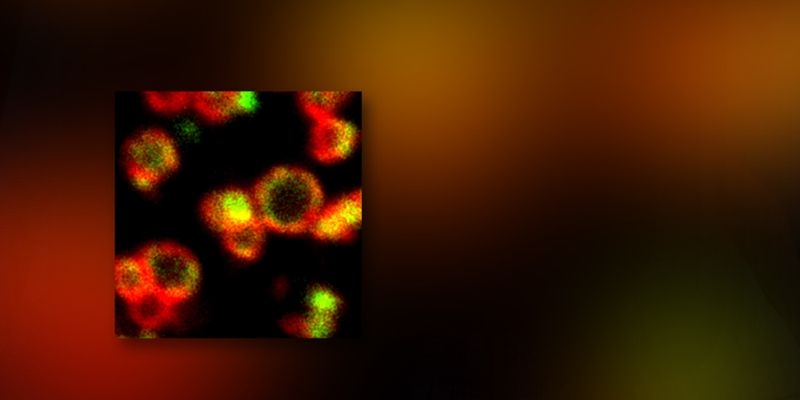
In the nasopharynx, sinus, and middle ear pneumococcus survive as a biofilm, a mode of growth ideal for long-term colonization, dissemination to tissues, and recalcitrant to antibiotic treatment. For biofilm development and dissemination, pneumococcus must sense host stimuli and respond in a coordinated fashion mediated by communication across bacterial cells. However, the mechanisms by which pneumococcus integrates and coordinates host signals, biofilms, and virulence are not well understood. In an article in Molecular Microbiology this month Rolando A. Cuevas, a doctoral student in the Hiller lab, describes virulence peptide 1 (VP1) a novel communication peptide employed by pneumococcus and critical for biofilms formation and pathogenesis.
The VP1 peptide was identified in a comparative genomics screen for communication molecules, and it was prioritized for further study because it is highly expressed during infection. Cuevas demonstrated that VP1 stimulates biofilm development on middle ear epithelial cells using a model adapted with Kevin Mason at Nationwide Children’s Hospital. Moreover, VP1 promotes dissemination and dramatically influences mortality in an animal model of infection. Finally, Cuevas determined that VP1 is controlled by a second communication system (Rgg/SHP), also activated by a secreted peptide. In this manner, this work reveal two novel communication peptides VP1 and SHP, as they both cycle through the extracellular milieu and influence gene expression in producing and neighboring cells.
The article was selected for the front cover of Molecular Microbiology. A long- term goals of the Hiller lab is to identify high-value targets for anti-pneumococcal therapies, development of diagnostics, and preventions of pneumococcal disease. To reach this goal the lab is compiling a dictionary of pneumococcal signals and studying these in the context of virulence and biofilms. Cuevas is currently studying the molecular components of the VP1 signaling pathway and its downstream effectors, and in doing so is likely to reveal novel signatures of pneumococcal pathogenesis.
Cuevas RA, Eutsey R, Kadam A, West-Roberts JA, Woolford CA, Mitchell AP, Mason KM, Hiller NL. A novel streptococcal cell-cell communication peptide promotes pneumococcal virulence and biofilm formation. Mol Microbiol. 2017 Aug;105(4):554-571. doi: 10.1111/mmi.13721. Epub 2017 Jun 23. PMID: 28557053