Uncovering the Hidden Secrets of Atlastin
The spontaneous fusion of separate lipid bilayer membranes proceeds slowly or not at all under typical conditions found inside of cells due to the high activation energy required for fusion, and yet it underlies major cellular processes like secretion and organelle maintenance. Cells accomplish this task through the use of specific fusion protein catalysts that draw membranes together and provide the energy necessary to disrupt two lipid bilayers so that the two can merge into one. Atlastin, the protein responsible for homotypic fusion of the endoplasmic reticulum (ER), is one such catalyst.
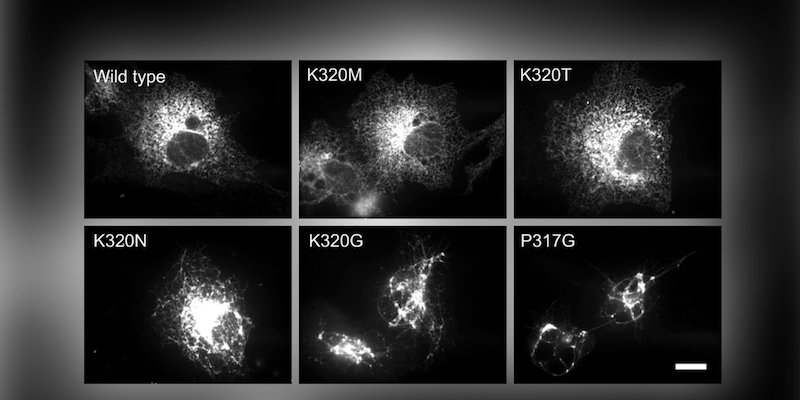
Recent work published in the Journal of Cell Biology by James Winsor, a fourth-year Ph.D. candidate in the Lee lab, has helped to shed light on how this poorly understood fusion catalyst functions. This study focused on the role of crossover, a large conformational shift that occurs during or just prior to fusion. By analogy to other fusion proteins crossover has been alternately suggested to either draw membranes close together, provide energy for fusion, or both. This confusion has arisen due to the complex multi-domain architecture of atlastin as well as the fact that it is a GTPase that directly ties nucleotide hydrolysis into the fusion cycle leading to a more complex mechanism.
This study used point mutations designed specifically to lower the amount of energy released during crossover, without otherwise impairing atlastin. Then, a series of fluorescence-based measurements reporting on the rates of formation and loss of the crossover state were made of each mutated version. The results showed, for the first time, that the energy released through crossover was directly related to the ability to fuse bilayers. This result showed that the energy for lipid bilayer disruption is provided by the crossover of atlastin molecules, and clarifies a central question about the atlastin fusion mechanism.