Chemists Test Museum Artifact To Identify Resin Ingredients
By Heidi Opdyke
Media Inquiries- Associate Dean for Communications, MCS
- 412-268-9982
- Director of Marketing and Communications, Carnegie Museum of Natural History
To learn about something old, curators at Carnegie Museum of Natural History turned to something new at Carnegie Mellon University.
Nicole Auvil is a doctoral researcher in the Department of Chemistry and a member of Mark Bier's research group. She and Bier used a "super-sniffer" they developed to test a sample of residue from a 2,500-year-old bronze cup used to hold incense.
"Studying the past is complicated and requires the work of all kinds of experts. I love collaborating with other people," said Lisa Saladino Haney, Egyptologist and assistant curator at Carnegie Museum of Natural History. "I find that the more ideas you have and the more methods for examining a particular question, the more interesting your answers/data will be. It always helps to see things from new perspectives."
Carnegie Museum of Natural History has more than 22 million objects in its care. Of those, the museum stewards approximately 5,000 objects and 17 individuals from the Nile Valley. Haney said most of the material comes from Egypt, including the cup.
Burning incense was a daily occurrence in ancient Egypt used in rituals, healing and daily life. Among the substances used were frankincense and myrrh as well as mixtures that included herbs, spices and gums made from tree resin.
This incense cup had been on display in the Museum's Walton Hall of Ancient Egypt but was removed a few months ago.
"This was a convenient time to take it out of the case and run some analytical testing on it. We are always looking for more information and data about the objects in our care," said Gretchen Anderson, head conservator at CMNH. "I am looking for any better way to preserve the objects, and Lisa is looking for more information that will help us understand the ancient cultures."
Opening Sept. 1, 2023, a new display, From Egypt to Pittsburgh: Sacred Scents, will showcase stories of Egyptian artifacts. Visitors will learn how the objects got from Egypt to Pittsburgh as well as why the objects were made in the first place and the care being given to them now. This is one of the first publicly visible steps toward the museum's plans to reimagine how it engages with visitors on the topic of ancient Egypt.
The cup was likely used in temple ceremonies, possibly as a storage container since the residue was still white and does not appear to have been burned, Haney said.
"Residue analysis is something that requires special expertise and equipment, so it isn't always possible to do," Haney said. "There are many different types of residue analysis. Often it can help you see how something was made, what it may have been made of — or in the case of a cup, what it may have originally contained — or how it was used."

An ultrasharp needle housed inside the "super-sniffer" emits a corona discharge to ionize a sample of the resin for testing.
For any material analysis the museum needs, Travis Olds is the point person. Olds is assistant curator of minerals in the Section of Minerals and Earth Sciences at CMNH. While his lab hosts a large assortment of materials characterization equipment, a mass spectrometer is not part of it.
Olds reached out to Mark Bier, a research professor in chemistry and director for the Center Molecular Analysis at Carnegie Mellon, to see if he could help study the cup.
Bier responded. With more than a dozen patents related to mass spectrometry, Bier and his lab are working to build improved instrumentation and methods to determine the mass of different molecules in a sample. Mass spectrometers — which measure the mass of a molecule after it converts the molecule to a gas-phase ion via an electric charge — are the workhorse of many labs and have been used to discover isotopes, record precise atomic weights and characterize molecules.
In Auvil's first semester, Bier offered up several building projects including one that had been unsuccessfully attempted by previous students. The challenge: figuring out how to reproducibly make ultrasharp needles out of tungsten metal and further developing a mass spectrometer ionization source with which to use them.
"That was a tricky task," she said. "At the time, we didn't know if it would be possible to make needles this sharp with such an intense geometry... and to make them reproducibly? I knew I was taking on a project that might not be successful."
The needle's job is to emit a highly energetic plasma called a corona discharge. The sharper the needle, the lower the voltage it requires do to this job. Corona discharge ionizes the ambient air (and whatever molecules it may contain) in a small region around the needle tip — a proton is added or one or more electrons are knocked off to create positive ions.
"We are wondering whether two ionization mechanisms are at play here," Bier said.
The ions are then sucked into a vacuum chamber and into a mass analyzer for mass-to-charge analysis and detection. For a linear ion trap mass analyzer — one of Bier's most impactful inventions — this is accomplished by measuring the point of radial ion ejection of each ion out of the trapping region. The instrument records the relative abundances of an ion’s mass-to-charge to produce a visual read-out called a mass spectrum.
Commercial needles used for atmospheric pressure chemical ionization typically have a tip radius greater than 14 microns at their sharpest point. Through an electrochemical etching process adapted from methods found in the literature, Auvil figured out how to make extremely sharp needles in-house that were 18 nm at their point by the start of her second semester.
"The first needles she showed me were fabulous with a beautiful parabolic tip," Bier said. "These needles turned out to be ultra-sharp, with a very desirable short tip and they were reproducible which was another goal of this aspect of the project. The needles were better than I had imagined and similar to the electron gun needles used in scanning electron microscopes."
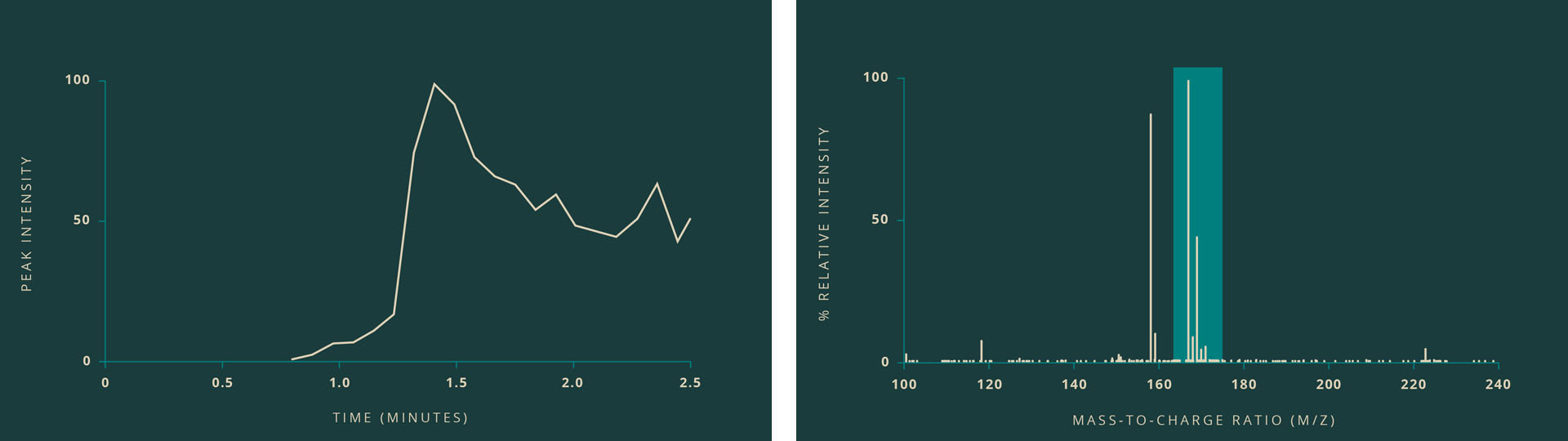
At left, a line graph shows the time and intensity of when a heated sample is presented to the sniffer. The intensity of the peak correlates with presence of the resin sample suggesting the molecule being measured is coming from the sample. At right, a mass spectrum shows peaks in different molecules or isotopes detected when the heated sample is "sniffed." The mass-to-charge ratio and isotopic distribution indicate that the sample may be Arabinoic acid.
She and Bier have filed a provisional patent on the device and a paper will soon be submitted. With the needle question resolved, the "super-sniffer" was put to work on many sample types.
So far, she has tested the device on medicine, air pollution, receipt paper coatings and even her own skin.
"Twenty minutes after drinking a cup of coffee, the sniffer can detect caffeine coming out of your pores," Auvil said. "My graduate career will include further development of this ionization source as well as adapting it for specific applications such as imaging, air quality monitoring, breath analysis and nicotine vape analysis."
In July, Haney, Olds, Anderson and Mostafa Sherif, a conservator working to preserve a wooden funerary boat in the museum's collection; along with Bier and Auvil walked the cup between the museum and Bier's lab. Protected in a container with silica gel to keep it maintained in a dry environment, it was the first time the cup left the museum in 30 years.
Before the testing could begin, Auvil tested the air of the room with all of its occupants to establish a background mass-spectrometry signal. Once that process was complete, she and Haney passed the cup underneath the needle as close as possible without touching it. Additional samples were taken when minute amounts of residue from the cup were scraped into a vial, heated and tested.
Auvil and Bier took a few days to analyze the data. With the first passes, there was not a strong signal from the cup itself. One recommendation was to store the cup in a sealed bag for a few days to let the volatile molecules build up and then try again. With the samples of the powder that were removed from the cup and heated, the researchers observed signals that appeared to correlate with the sample.
 "Taking into account the molecular weight, isotopic distribution, the software-suggested molecular formulae, and the literature, we were able to tentatively identify this molecule as Arabinonic acid," Auvil said. Also known as Arabic acid, the molecule can be found as a degradation product of the simple sugar arabinose, which can be found in Arabic gum (at right) from acacia trees and in cannabis sativa, both native to Egypt.
"Taking into account the molecular weight, isotopic distribution, the software-suggested molecular formulae, and the literature, we were able to tentatively identify this molecule as Arabinonic acid," Auvil said. Also known as Arabic acid, the molecule can be found as a degradation product of the simple sugar arabinose, which can be found in Arabic gum (at right) from acacia trees and in cannabis sativa, both native to Egypt.
"Although exciting, we need to do more experiments to gain confidence in this identification," Bier said.
The museum is still working to determine what next steps for the cup will be, which could include using an X-ray fluorescence technology to test the source of the bronze and to also conduct Raman and Fourier-Transform Infrared spectroscopicanalyses (FTIR) the residue.
"I had never collaborated with the scientists at CMNH even though they are only two blocks away in location," Bier said. "It was just such a unique and valuable sample and the people at the museum were equally excited about the possibilities of our work. They would like us to look at more ancient samples in the future."
