Carnegie Mellon Research Shows How Wildfires May Have Larger Effects on Cloud Formation and Climate Change than Previously Thought
By Ben Panko
As the frequency and size of wildfires continues to increase worldwide, new research from Carnegie Mellon University scientists shows how the chemical aging of the particles emitted by these fires can lead to more extensive cloud formation and intense storm development in the atmosphere. The research was published online today in the journal Science Advances.
"The introduction of large amounts of ice-nucleating particles from these fires can cause substantial impacts on the microphysics of clouds, whether supercooled cloud droplets freeze or remain liquid, and the propensity of the clouds to precipitate," said Ryan Sullivan, associate professor of chemistry and mechanical engineering. Understanding these impacts is a key factor in accurately modeling Earth's climate and how it could continue to change.
Building on research Sullivan's team in the Center for Atmospheric Particle Studies published last year, the authors collected a variety of different plant materials, burned them and analyzed the particles emitted in the smoke. In particular, the team was interested in ice nucleating particles, rare types of particles that can catalyze ice crystal formation in the atmosphere at higher than usual temperatures and thus greatly affect climatic processes, including cloud formation and whether a cloud precipitates or not. In fact, most precipitation over land starts from ice-containing clouds.
While it was already widely known that particles freshly emitted by the burning of biomass – like tall grasses, shrubs, and trees – can impact ice nucleation greatly, Sullivan's team was interested in finding out the effects of these particles as they traveled for days and weeks in the atmosphere and experienced chemical aging. With a specialized chamber reactor, mass spectrometers, electron microscopy, and an innovative microfluidic droplet freezing technique, the researchers analyzed the particles emitted by the burning of various types of plant material as occurs in wildfires and prescribed burns, and simulated the aging processes these particles would undergo in the atmosphere.
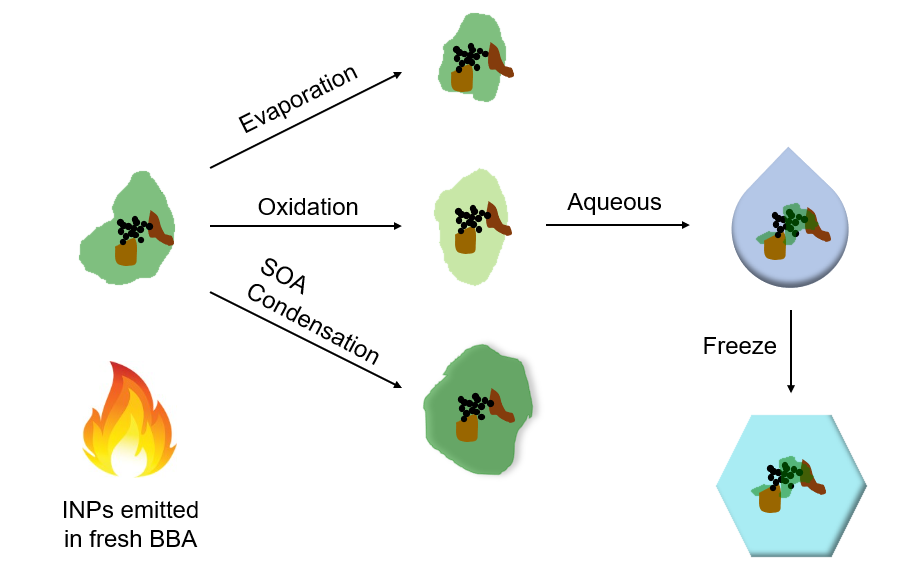
A diagram showing the various aging processes of biomass-burning aerosol in the atmosphere.
Typically, ice nucleating particles lose their potency as they age in the atmosphere, but in this study, the researchers found that the ice nucleating ability of particles emitted by burning biomass actually increased as they experienced simulated atmospheric aging. This represents a very different framework for considering how the climate-forcing properties of a major episodic source of particles evolve in the atmosphere.
"This is because atmospheric aging drives the loss of particle coatings initially present on the smoke particles that conceal the ice-active surface sites," Sullivan explained. "Those sites are the mineral particles produced by the biomass fuel combustion itself that we reported last year in the Proceedings of the National Academy of Sciences."
The data from this study could have a major impact on future research into wildfires and climate change, said Lydia Jahl, who recently received her Ph.D. in chemistry from Carnegie Mellon in Sullivan's group.
"We estimated that burning just one square meter of grassland could influence the concentration of ice nucleating particles in hundreds of thousands of cubic kilometers of the atmosphere," Jahl said. "Climate modelers could use our data further to determine how wildfire emissions influence the balance of incoming solar radiation and outgoing terrestrial radiation, among other cloud properties."
Other authors on this study were Thomas A. Brubaker, Bailey B. Bowers and William D. Fahy of Carnegie Mellon; Micahel J. Polen, now of McDaniel College; Leif G. Jahn, now of the University of Texas at Austin; Kerrigan P. Cain, now of NASA Glenn Research Center; and Sara Graves, now of the University of California, Los Angeles.
Funding for this study was provided by grants from the National Science Foundation (CHE-1554941, AGS-1552608, and CBET-1804737).