The Future of Human Healing Lies in the Brain of a Starfish
By Adam Dove
The incredible benefits of stem cell therapy have been widely known for decades. It can alleviate the pain of arthritis and help patients heal exponentially faster after surgery. But stem cell therapies are prohibitively expensive—to the tune of $100,000 to $200,000 per patient. Because of the exorbitant cost, these life-changing treatments are far out of range of the vast majority of those who need them. But thanks to Professor and Head of Biological Sciences Veronica Hinman and Professor of Chemical Engineering Kris Noel Dahl, stem cell therapy is about to get a whole lot cheaper. And the key to it all lies in the incredible regenerative powers of starfish.
“Many species of animals, including starfish, have extraordinary capacities to regenerate and can reform all lost body parts following traumatic injury,” says Hinman. “This capacity derives in part from their natural abilities to dedifferentiate and reprogram cells. Understanding how cellular reprogramming occurs in nature has great potential for scientists to translate these findings to human cell cultures to identify cheap and robust ways to generate stem cells.”
Stem cells are the body’s raw material, able to become any type of cell the body needs: muscle cells, skin cells, blood cells and more. In humans, when stem cells differentiate into these other cells, they are unable to change back, or dedifferentiate. And they most definitely can’t turn into a different kind of cell. But this is not the case with starfish. Notably, if a starfish loses one of its arms, it can grow it back just the same. This is due to the starfish’s unique cells, which can dedifferentiate themselves from skin or muscle cells back into stem cells. While this regenerative capability in itself is incredible, Hinman’s preliminary research has shown an even more incredible ability.
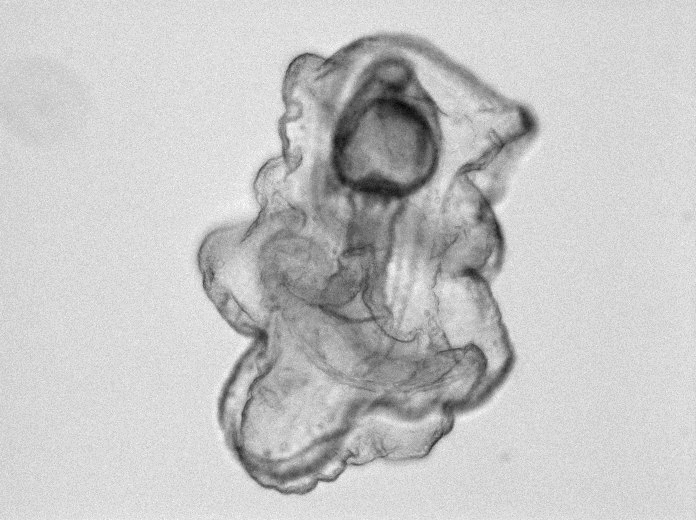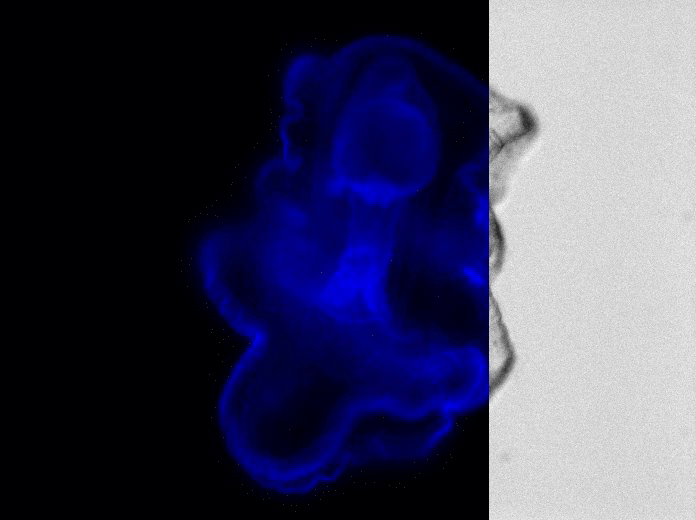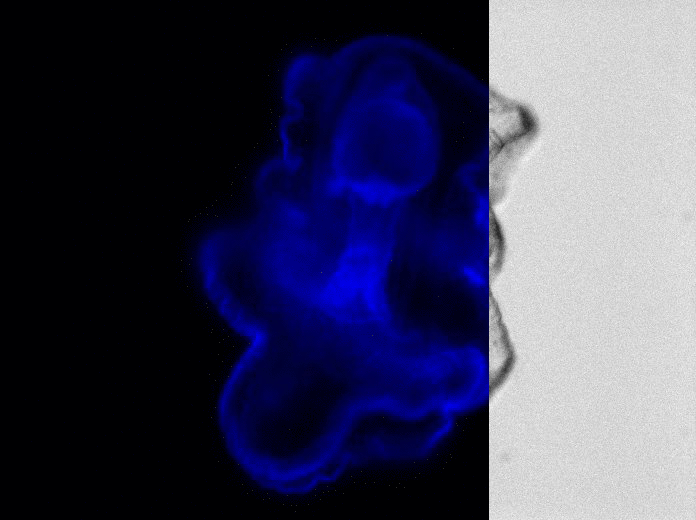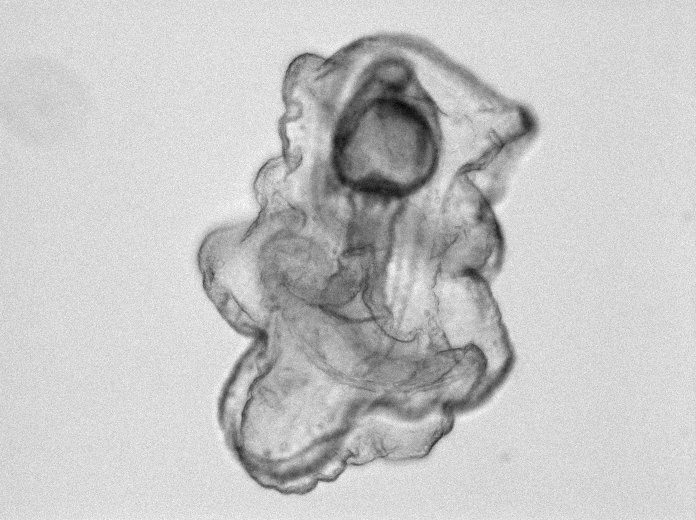
Starfish Embro (Patiria Miniata) twelve days after fertilization.
“In the larval state, starfish have a distinctive head that contains their brain,” says Dahl. “If the head is removed or damaged, the differentiated cells that are definitely not neural cells will dedifferentiate, crawl up to the head region, and regrow into neurons. To not only do this in the larval state, but to regrow something as complex as a brain—this is an amazing regenerative capability.”
Supported by funding from the DSF Charitable Foundation block grant program, Hinman and Dahl are working to understand just what in the starfish causes their cells to do this. While Hinman is focused on the fundamental science, Dahl’s lab is delving into the structure of the cells, cell crawling and the biomechanics of cellular regeneration.
The Hinman lab uses the larvae of starfish to understand the genomic control of development and cell fate decisions. They have developed tools to engineer transgenic larvae, measure gene expression and chromatin changes, and track cells during specification. With these tools, Hinman and her team have shown that following amputation, cells at the wound site turn on pluripotency factors and undergo cell fate reprogramming to regenerate lost tissue.
“While regenerative medicine is great, there’s still a lack of understanding of the fundamentals that govern how cells respecify themselves,” says Dahl. “The hope is that by studying a model organism like the starfish and combining what we learn with our knowledge of human stem cells, we can use comparative genomics to understand the gene expression that allows starfish cells to respecify their programming.”
To do this, Dahl is creating an artificial model of the starfish’s larval system to map the cells as they crawl to their new destination. With this artificial model, Dahl and her team can manipulate the chemical and mechanical factors that exist in the starfish embryo, blocking them one at a time until they find exactly what it is that tells the cells to dedifferentiate back into stem cells, crawl up to the brain region and become neuronal tissue. Once this factor has been isolated, the goal is to then be able to apply it to human cells, to tell those cells to dedifferentiate so they can become whatever the patient needs.
Current therapies require stem cells to be harvested from a patient, then cultured over the course of days, in order to have enough to be reinjected back into the patient to help speed healing. But with this new method, cells could be taken from any part of the body, dedifferentiated back into stem cells, then re-differentiated into therapeutic cells. This could make the process of preparing stem cell therapies faster, easier and, most importantly, cheaper.
“If you could reduce stem cell therapy from $200,000 to $1,000—it would touch nearly every person’s life,” says Dahl. “Surgeons could include a stem cell injection with every major or minor surgery, helping patients heal 100 times faster. It’s quick healing, it’s reduced scarring: This could be like penicillin. I see it becoming the standard of care in the next ten years.”