NIH Eyes New Use for Old Technology
An NMR reactor built at CMU in the 1990s may help influence the future of medicine
Technology created by Carnegie Mellon University more than 25 years ago is having a resurgence.
The Laboratory of Cardiac Energetics — a research lab within the National Institutes of Health's (NIH) National Heart, Lung, and Blood Institute — is looking to revive a nuclear magnetic resonance (NMR) technology that could influence medicine, from preventative to prescriptive and everything in between.
Chemical Engineering Professor Michael Domach and former doctoral students developed the technology in the early 1990s in collaboration with former faculty members Alan Koretsky and the late Elizabeth Jones.
By using the NMR technology, the NIH lab hopes to better understand how the molecules within cells are used to transfer and store energy through analyzing a microorganism called Paracoccus denitrificans. What makes the microbe so attractive to researchers is how similar it is to the mitochondria, which regulates respiration and energy production within cells.
"What they can see then is what kind of molecules the cells are using to manage their energy metabolism," Domach said. "For those folks, it's all about the mitochondrial model and understanding how that sub-organelle works in heart and other cells."
Learning how, exactly, a microorganism so closely related to the mitochondria manages to survive events that should kill it gives researchers the opportunity to better understand things like ischemia, heart attacks and oxygen loss.
NMR technology is most commonly encountered in magnetic resonance imaging, better known as MRI. Electromagnetic radiation (radio frequency energy) is transmitted into a subject and offers a picture of what is going on inside that subject. In an MRI, the electromagnetic radiation produces a picture from inside a patient's body: for example, torn cartilage or tumors. Domach's focus is on NMR spectroscopy — using a reactor to identify the abundance of different molecules within a cell and how they react in time.
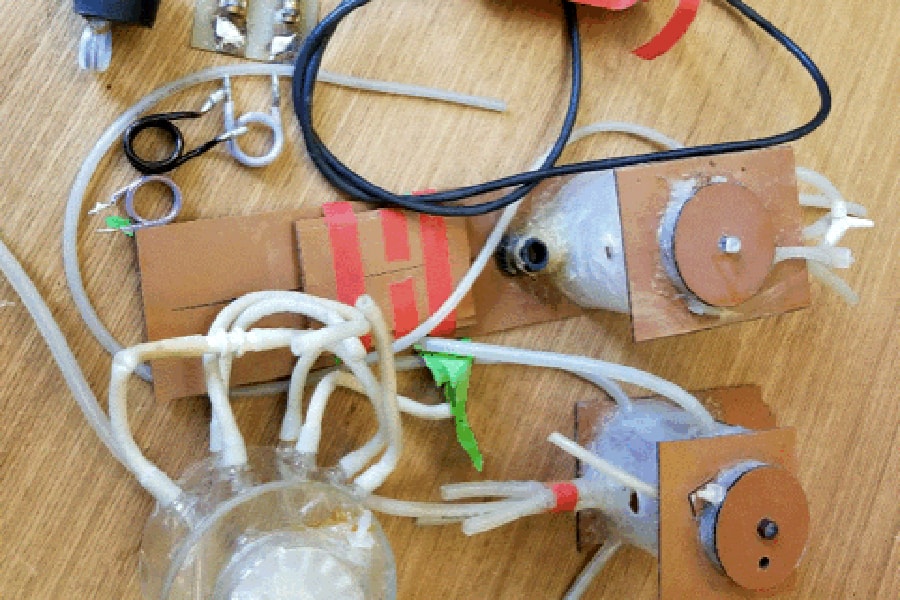 Disassembled parts of NMR spectroscopy reactor. The National Institutes of Health is looking to revive a nuclear magnetic resonance technology that could influence medicine.
Disassembled parts of NMR spectroscopy reactor. The National Institutes of Health is looking to revive a nuclear magnetic resonance technology that could influence medicine.
Here's how NMR works: When a subject — whether a chemical sample or a person — is hit with a burst of radio frequency energy, molecules within the subject that have "spin" (properties of magnetism) absorb the energy and get excited. Those molecules shoot the energy back out at a frequency that depends in part on the type of molecule. The energy being shot back at different frequencies creates spectra, or pictures, of the molecules present and their relative abundance at that instant. Once the molecules release that energy, they eventually relax back to the state they were in before the radiation burst. The process is repeated numerous times to gain a full readable spectrum by combining data from each individual radiation burst.
"The molecules have to sort of get back to what they were doing" before they can be hit with another burst of radiation, Domach said. "So that puts an automatic limitation on how fast you can get information out."
Because of this, NMR spectroscopy was traditionally ineffective in following molecular changes within a sample over time. During the time it takes for a sample to calm down, the molecules could be doing things that researchers can't observe. In the early 1990s, Domach, his then-Ph.D. students, Anthony Meehan and Chris Castro, and Koretsky addressed this shortcoming by developing a spectroscopy reactor that uses chemostat system principles described by 1965 Nobel Prize winner Jacques Monod.
A chemostat is a controlled chemical composition system in which researchers manipulate the growth rate and abundance of cells by removing some cells and replacing them with new cells at a predetermined and constant rate. This creates what Domach calls a "steady-state little ecosystem." In essence, every cell in a chemostat is growing at the same rate as chosen by the researchers.
"We were trying to replicate what a microbial physiologist would have done on the lab bench," he said.
Domach combined chemostat system principles with fluid mechanics — how fluids move in response to forces on them — to create an NMR spectroscopy reactor that sidesteps the usual wait-time needed between bursts of radiation shot at a sample. The result was that a researcher can choose an initial growth rate for the cells, scan them for a baseline and then perturb the system to watch the cells' metabolic adaptations and responses.
With the chemostat system in Domach's reactor balancing the cells' environment, researchers can introduce the desired variables to see how the cells' molecules respond. The real benefit, though, is the "real-time" view researchers get of how molecules work as time passes. Instead of waiting for molecules to recover, researchers have a constant fresh supply of cells with molecules ready to offer up information.
As innovation continues to advance science, older technologies still have a part in conversations.
"The [NIH lab] came across this technology and thought it might be the recipe to get done what they're trying to do." Domach said. The NIH work is in its early stages, but Domach said he is excited to see where the research might lead next.