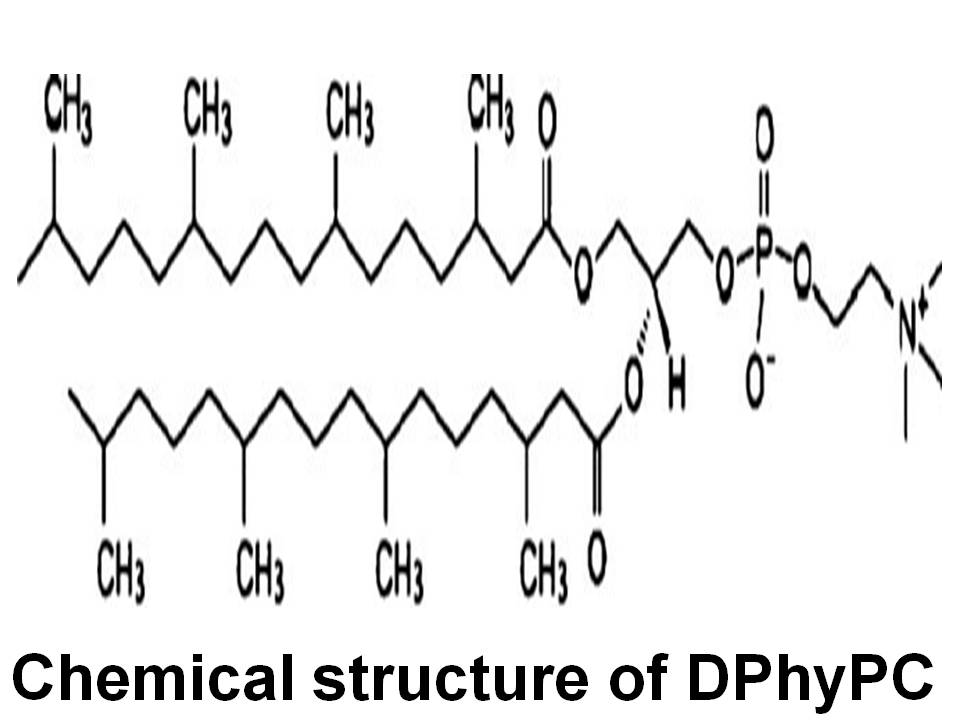
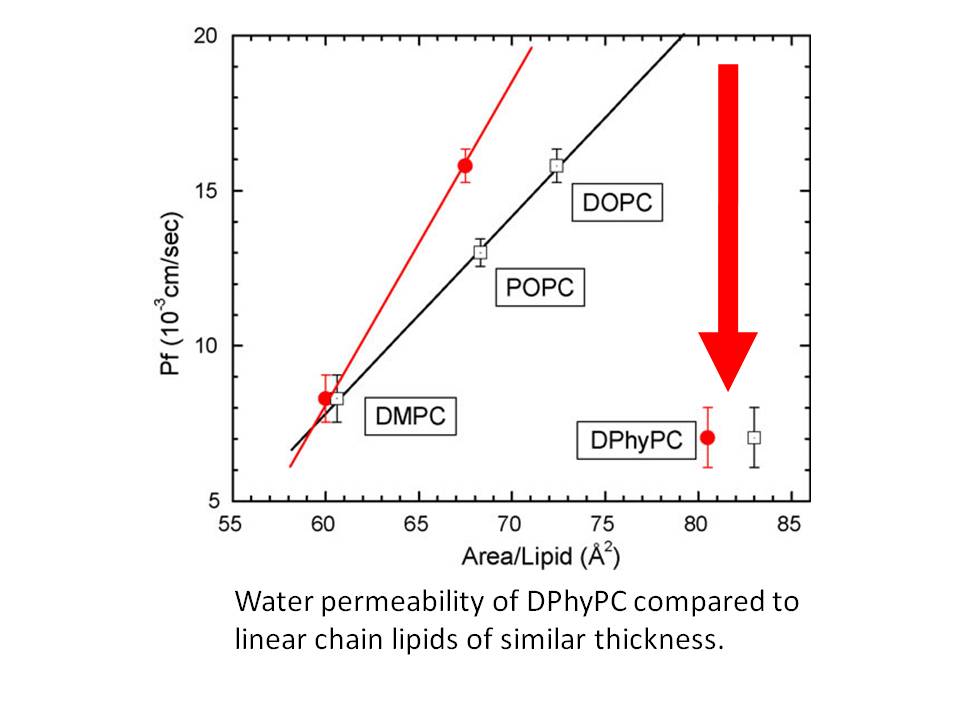
Questions: How does the presence of a branched chain on the lipid affect the structure of a lipid membrane and its water permeability? How does a branched chain affect the properties of a lipid membrane? We studied the branched chain lipid, diphytanoylPC, and found that its thickness is nearly identical to that of the well-studied lipid DOPC, but its area is much larger mainly due to its larger molecular volume. Its water permeability is much smaller than predicted by our 3-slab permeability model, indicating that water does not permeate as well through a hydrocarbon region with branched chains.
Structure and water permeability of fully hydrated diphytanoylPC
Stephanie Tristram-Nagle1, Dong Joo Kim2, Nadia Akhunzada3, Norbert Kucerka4,
John C. Mathai5, John Katsaras4, Mark Zeidel5, John F. Nagle1,2
1 Biological Physics Group, Physics, Carnegie Mellon University, Pittsburgh, PA, United States
2 Biological Sciences, Carnegie Mellon University, Pittsburgh, PA, United States
3 Douglass College, Rutgers University, New Brunswick, NJ, United States
4 Canadian Neutron Beam Centre, National Research Council, Chalk River, Ontario K0J1J0, Canada
5 Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, United States
a b s t r a c t
Diphytanoylphosphatidylcholine (DPhyPC) is a branched chain lipid often used for model membrane studies, including peptide/lipid interactions, ion channels and lipid rafts. This work reports results of volume measurements, water permeability measurements Pf, X-ray scattering from oriented samples, and X-ray and neutron scattering from unilamellar vesicles at T=30◦C. We measured the volume/lipid VL = 1426±1Å3. The area/lipid was found to be 80.5±1.5Å2 when both X-ray and neutron data were combined with the SDP model analysis (Kuˇcerka, N., Nagle, J.F., Sachs, J.N., Feller, S.E., Pencer, J., Jackson, A., Katsaras, J., 2008. Lipid bilayer structure determined by the simultaneous analysis of neutron and X-ray scattering data. Biophys. J. 95, 2356–2367); this is substantially larger than the area of DOPC which has the largest area of the common linear chain lipids. Pf was measured to be (7.0±1.0)×10−3 cm/s; this is considerably smaller than predicted by the recently proposed 3-slab model (Nagle, J.F., Mathai, J.C., Zeidel, M.L., Tristram-Nagle, S., 2008. Theory of passive permeability through lipid bilayers. J. Gen. Physiol. 131, 77–85). This disagreement can be understood if there is a diminished diffusion coefficient in the hydrocarbon core of DPhyPC and that is supported by previous molecular dynamics simulations (Shinoda, W., Mikami, M., Baba, T., Hato, M., 2004. Molecular dynamics study on the effects of chain branching on the physical properties of lipid bilayers. 2. Permeability. J. Phys. Chem. B 108, 9346–9356). While the DPhyPC head–head thickness (DHH = 36.4 Å), and Hamaker parameter (H= 4.5×10−21 J) were similar to the linear chain lipid DOPC, the bending modulus (KC = 5.2±0.5×10−21 J) was 30% smaller. Our results suggest that, from the biophysical perspective, DPhyPC belongs to a different family of lipids than phosphatidylcholines that have linear chain hydrocarbon chains. © 2009 Elsevier Ireland Ltd
 |
 |
We welcome you to read the article. Click here.
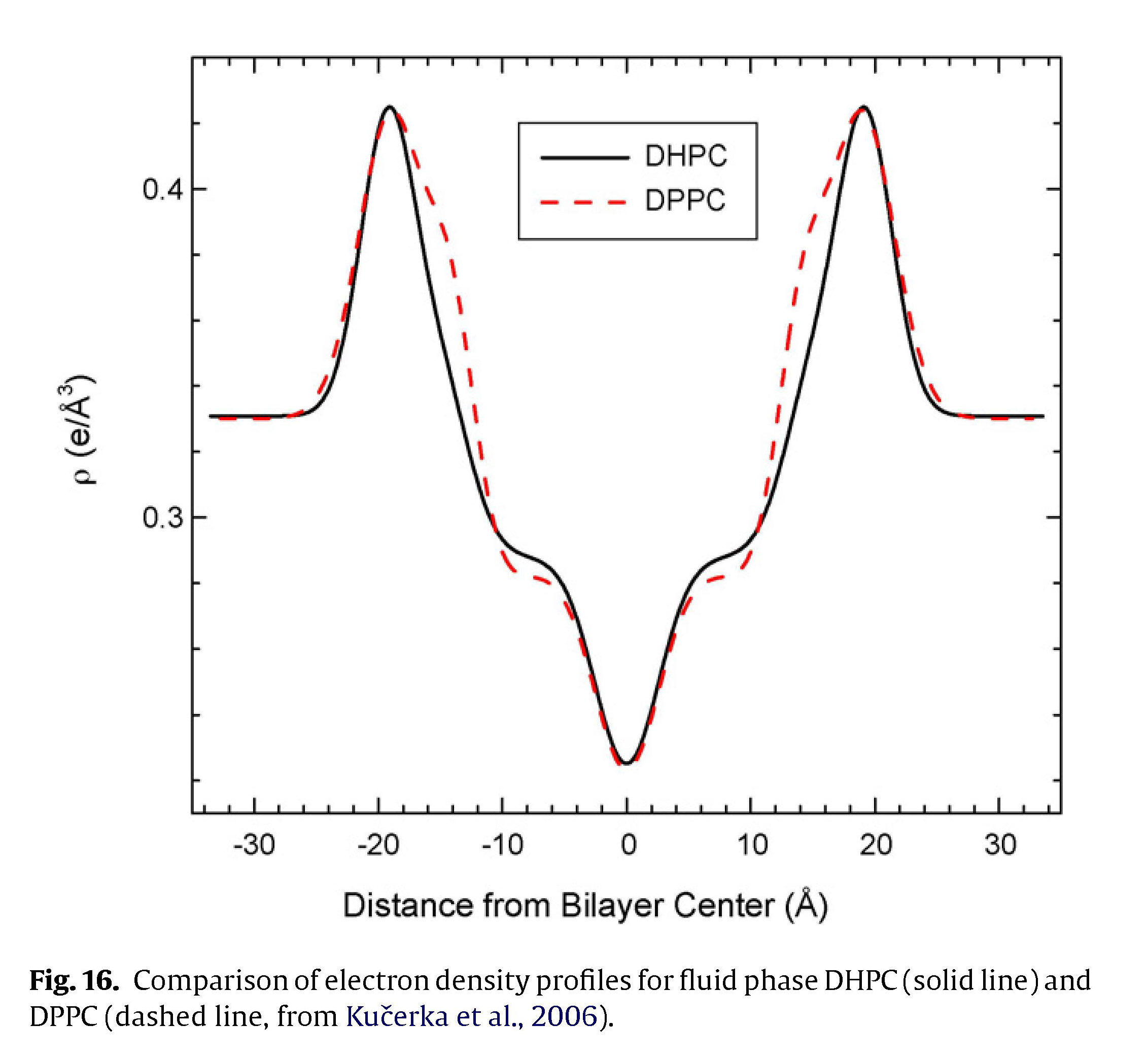
Questions: How does the presence of a carbonyl group on the lipid chain affect the structure of a lipid membrane? I.e., what is the difference between a lipid with an ester linkage (with carbonyl) and an ether linkage (no carbonyl)? We compared the structures of DPPC (ester linkage) and DHPC (ether linkage) to understand how the carbonyl group affects the electron density profile (structure).

Effects of ether vs. ester linkage on lipid bilayer structure and water permeability
S. Deren Guler1, D. Dipon Ghosh2, Jianjun Pan1, John C. Mathai3, Mark L. Zeidel3,
John F. Nagle1,2, Stephanie Tristram-Nagle1
1 Department of Physics, Carnegie Mellon University, Pittsburgh, PA 15213, USA
2 Department of Biological Sciences, Carnegie Mellon University, Pittsburgh, PA 15213, USA
3 Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Cambridge, MA 02139, USA
a b s t r a c t
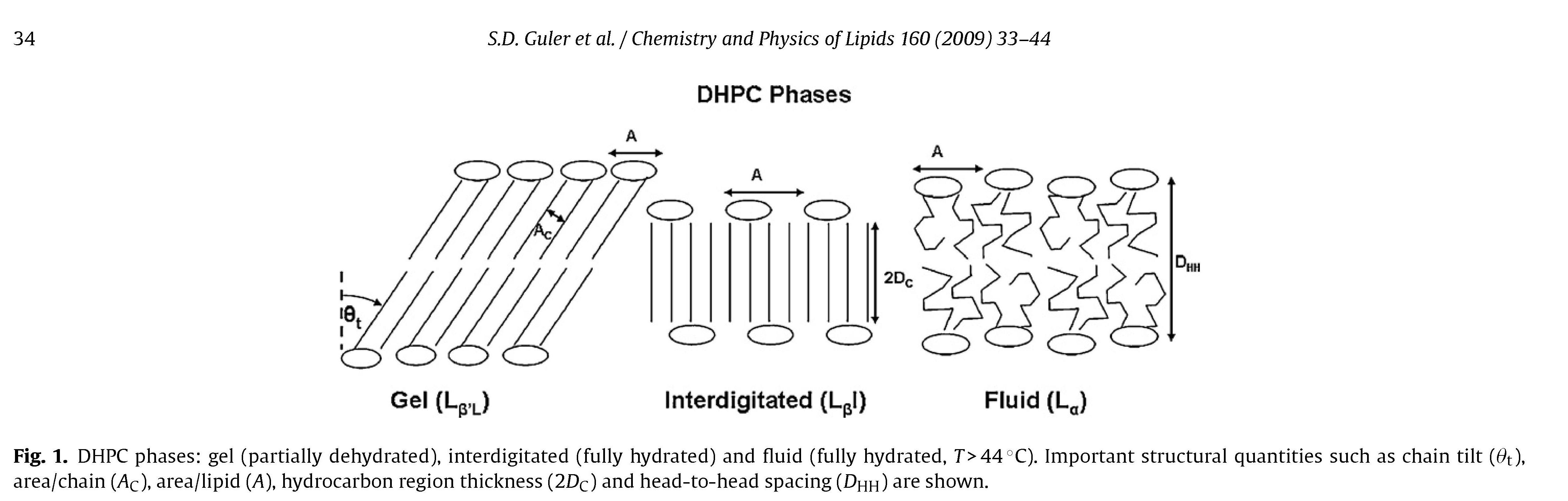
The structure and water permeability of bilayers composed of the ether-linked lipid, dihexadecyl-phosphatidylcholine (DHPC), were studied and compared with the ester-linked lipid, dipalmitoyl-phosphaditdylcholine (DPPC). Wide angle X-ray scattering on oriented bilayers in the fluid phase indicate that the area per lipid A is slightly larger for DHPC than for DPPC. Low angle X-ray scattering yields A = 65.1Å^2 for DHPC at 48 ◦C. LAXS data provide the bending modulus, Kc = 4.2×10^−13 erg, and the Hamaker parameter H=7.2×10^−14 erg for the van der Waals attractive interaction between neighboring bilayers. For the low temperature phases with ordered hydrocarbon chains, we confirm the transition from a tilted L-Beta
gel phase to an untilted, interdigitated L-BetaI phase as the sample hydrates at 20 ◦C. Our measurement of water permeability, Pf = 0.022 cm/s at 48 ◦C for fluid phase DHPC is slightly smaller than that of DPPC (Pf = 0.027 cm/s) at 50 ◦C, consistent with our triple slab theory of permeability. © 2009 Elsevier Ireland Ltd
We welcome you to read the article. Click here.
These structures are included in a table summarizing all of lipid structures we have obtained to date. To see the table, click here.
|
Precise Structures