Former lab member, Tanvi Jakkampudi, wins Student Research Paper Award at the Cornell High Energy Synchrotron Source (CHESS), June, 2024.
Ithaca, NY–Tanvi Jakkampuddi won the award for best Student Research Paper at the CHESS user's meeting in June, 2024. Tanvi's paper is: 2023. Lung SPLUNC1 Peptide Derivatives in the Lipid Membrane Headgroup Kill Gram-Negative Planktonic and Biofilm Bacteria. Biomacromolecules 24:2904-2815. The authors are: Jakkampudi, T., Lin, Q., Mitra, S., Vijai, A., Qin, W., Kang, A., Chen, J., Ryan, E., Wang, R., Gong, Y., Heinrich, F., Song, J., Di, Y.-P., Tristram-Nagle, S. Tanvi presented her work as a remote talk at the CHESS User's meeting. She also received a cash prize of $500.
Congratulations Tanvi!
Prof. Stephanie Tristram-Nagle Wins Charles E. Kaufman Award, December, 2010.

PITTSBURGH –The Pittsburgh Foundation announced that Carnegie Mellon University research professor Stephanie Tristram-Nagle was awarded the third annual Charles E. Kaufman Award of $50,000 for her ground-breaking research in lipid membranes, the underlying structure of all living cell membranes, which may one day lead to a breakthrough in the treatment of AIDS.
The award is presented annually to an honoree that demonstrates "substantial contributions to science for both the betterment and understanding of human life." The late Mr. Charles Kaufman established the award in 2008 at The Pittsburgh Foundation "to promote a better and fairer world by supporting those that can make a difference with science." Mr. Kaufman passed away in September at the age of 97.
To read the full publicity please follow these links: Mellon College of Science, CMU and Pittsburgh Tribune-Review.
Prof. Tristram-Nagle is named first Gluckstern lecturer at University of Massachusetts, Amherst, October, 2010.

Prof. Stephanie Tristram-Nagle was recently named the first Gluckstern lecturer by Profs. V. Adrian Parsegian and Tony Dinsmore who are co-teaching "The Gluckstern Lectures in Biological Physics", an advanced-topics graduate course (see Series.pdf for more information). Prof. Parsegian is shown at left with Prof. Tristram-Nagle before the second of two classes which shegave during her 5-day visit to U. Mass. from October 2 to 7, 2010. In addition to two classes, Prof. Tristram-Nagle also led the grad students in x-ray data collection and analysis of lipid samples under osmotic pressure. The lab class culminated with construction of an electron density profile of the lipid DPPC using the students' data. In addition, Prof. Tristram-Nagle spoke at Prof. Parsegian's scientific writing class about the mechanics of writing scientific publications. On the last day of her visit, Prof. Tristram-Nagle received the Gluckstern plaque (see below). 
Profs. Tristram-Nagle and Nagle find key HIV protein makes cell membranes bend more easily.
 Carnegie Mellon University scientists have made an important discovery that aids the understanding of why HIV enters immune cells with ease. The researchers found that after HIV docks onto a host cell, it dramatically lowers the energy required for a cell membrane to bend, making it easier for the virus to infect immune cells. The finding, in press in Biophysical Journal, will provide vital data to conduct future computer simulations of HIV dynamics to help further drug discovery and prevent deadly infections. Carnegie Mellon University scientists have made an important discovery that aids the understanding of why HIV enters immune cells with ease. The researchers found that after HIV docks onto a host cell, it dramatically lowers the energy required for a cell membrane to bend, making it easier for the virus to infect immune cells. The finding, in press in Biophysical Journal, will provide vital data to conduct future computer simulations of HIV dynamics to help further drug discovery and prevent deadly infections.
"We found that HIV fusion peptide dramatically decreases the amount of energy needed to bend a cell-like membrane," said Stephanie Tristram-Nagle, associate research professor of biological physics at Carnegie Mellon. "This helps membranes to curve, a necessary step for HIV to fuse with an immune cell as it infects it."
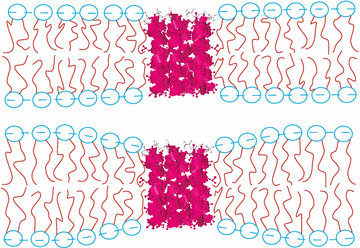
The Carnegie Mellon scientists used X-rays to study how HIV fusion peptide (part of a larger protein) affected the energy of manufactured lipid bilayers made to mimic normal cell membranes. Lipid bilayers provide a protective barrier for the cell against intruders, yet also contain molecules to recognize and communicate with other cells or get nutrients. Cells also communicate with one another via small, membrane-bound vesicles that contain proteins or other molecular cargo. When delivering their goods, vesicles from one cell fuse with the outermost membrane of another cell to form a series of hybrid structures called fusion intermediates.
Through evolution, viruses have also become skilled at fusing with cells to unload their genetic contents, which turn host cells into virus-producing factories. In the case of HIV, a molecule called gp120 initially helps the virus lock onto its host T cell, a cell critical for maintaining immunity. Another protein - gp41 - then enables HIV to penetrate a T-cell membrane. Fusion takes place specifically through a short stretch of gp41 called fusion peptide 23, or FP-23 for short. Prior studies have shown that FP-23 fuses with, and can even break apart, blood cells and other man-made, cell-like structures called liposomes.
FP-23 likely plays several roles in viral fusion, according to the researchers. One role already suspected is that FP-23 attaches to its T cell victim to facilitate a change in the shape of gp41, which in turn drives uptake of HIV RNA and proteins by the T cell. But the Carnegie Mellon work suggests that FP-23 plays another, equally important function - reducing the free energy of curved fusion intermediates. These fleeting shapes arise and disappear as HIV enters a T cell.
Normally, a cell membrane resists bending. Scientists can quantify the energy needed to overcome this resistance. The Carnegie Mellon team found that FP-23 reduces the energy required to penetrate an artificial cell membrane by up to 13 fold, depending on the thickness of that membrane.
"Reducing this energy should help explain in part how HIV infection occurs so readily," said Tristram-Nagle. "Our findings definitely will change how theoreticians think about virus-cell interactions. This same phenomenon could provide a general way that viruses use to infect cells, so it will be exciting to look at other viral systems with our experimental method," she said.
Many different viruses could enter cells by efficiently lowering the energy required to penetrate a cell's outer membrane, according to Tristram-Nagle and her collaborator, John Nagle, professor of physics and biological sciences at Carnegie Mellon.
The Carnegie Mellon scientists used X-rays to detect the effect of FP-23 on lipid bilayers that mimic cell membranes. Lipid bilayers form different phases that change with temperature, but the "fluid" phase is the most biologically relevant. Using X-ray diffuse scattering, the team quantified structural properties of different lipid bilayers seeded with FP-23 peptides. The lipid bilayers varied in their thicknesses, which affects the stiffness of cell membranes.
The research was conducted at Cornell University's CHESS synchrotron, which provides a high-intensity source of X-rays for various studies. In their next trip to this facility, the team plans to study FP-23 together with cholesterol, a molecule known to modulate the stiffness of cell membranes.
The work was supported by the National Institute of General Medicine. The Cornell High Energy Synchrotron Source is funded by the National Science Foundation.
To view the Biophysical Journal article visit HIV.pdf.
For more on this research, see www.cmu.edu/mcs/about-mcs/news/030328-nagles.html. |
|
News and Events




 Carnegie Mellon University scientists have made an important discovery that aids the understanding of why HIV enters immune cells with ease. The researchers found that after HIV docks onto a host cell, it dramatically lowers the energy required for a cell membrane to bend, making it easier for the virus to infect immune cells. The finding, in press in Biophysical Journal, will provide vital data to conduct future computer simulations of HIV dynamics to help further drug discovery and prevent deadly infections.
Carnegie Mellon University scientists have made an important discovery that aids the understanding of why HIV enters immune cells with ease. The researchers found that after HIV docks onto a host cell, it dramatically lowers the energy required for a cell membrane to bend, making it easier for the virus to infect immune cells. The finding, in press in Biophysical Journal, will provide vital data to conduct future computer simulations of HIV dynamics to help further drug discovery and prevent deadly infections.