Peptides in Membranes

Membrane Structure and Interactions Lab (MSIL)
Research Highlights:
Water Permeability Through Membranes
Questions: What is the location of an antimicrobial peptide, alamethicin, in a membrane? How is the barrel stave formed by this transmembrane peptide affected by the thickness of the lipid bilayer? How does the lipid bilayer thickness affect the barrel stave? How does alamethicin affect the elastic properties of the lipid membrane?
Alamethicin Aggregation in Lipid Membranes J Membrane Biol (2009) 231:11–27 Jianjun Pan, Stephanie Tristram-Nagle and John F. Nagle Physics Department, Carnegie Mellon University, Pittsburgh, Pennsylvania 15213, USA
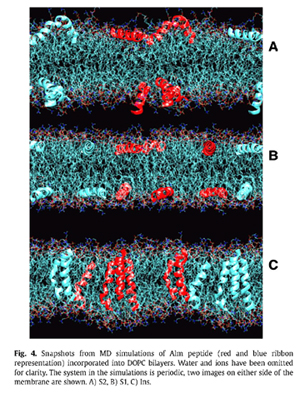
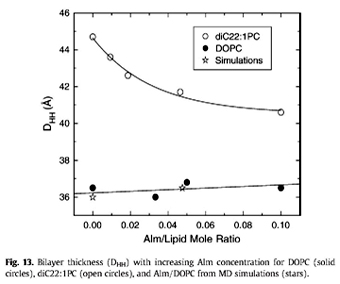
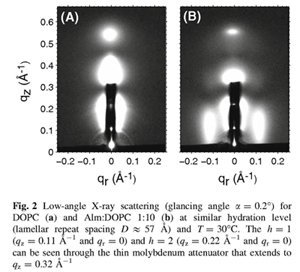
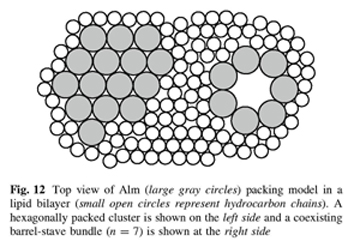
a b s t r a c t X-ray scattering features induced by aggregates of alamethicin (Alm) were obtained in oriented stacks of model membranes of DOPC(diC18:1PC) and diC22:1PC. The first feature obtained near full hydration was Bragg rod in-plane scattering near 0.11 Å^-1 in DOPC and near 0.08 Å^-1 in diC22:1PC at a 1:10 Alm:lipid ratio. This feature is interpreted as bundles consisting of n Alm monomers in a barrel-stave configuration surrounding a water pore. Fitting the scattering data to previously published molecular dynamics simulations indicates that the number of peptides per bundle is n = 6 in DOPC and n C 9 in diC22:1PC. The larger bundle size in diC22:1PC is explained by hydrophobic mismatch of Alm with the thicker bilayer. A second diffuse scattering peak located at qr & 0.7 Å^-1 is obtained for both DOPC and diC22:1PC at several peptide concentrations. Theoretical calculations indicate that this peak cannot be caused by the Alm bundle structure. Instead, we interpret it as being due to two-dimensional hexagonally packed clusters in equilibrium with Alm bundles. As the relative humidity was reduced, interactions between Alm in neighboring bilayers produced more peaks with three dimensional crystallographic character that do not index with the conventional hexagonal space groups.
We welcome you to read the article - click here. |
|
||
 We welcome you to read the article - click here. We welcome you to read the article - click here. |
 |
|

 Alamethicin in lipid bilayers: Combined use of X-ray scattering and MD Simulations
Alamethicin in lipid bilayers: Combined use of X-ray scattering and MD Simulations