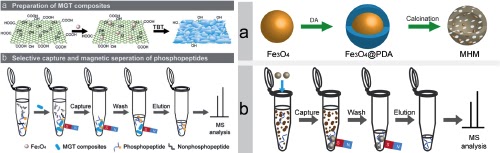
Nanomaterials for peptide capture
We designed and constructed a novel graphene composite affinity material consisting of graphene scaffolds, Fe3O4 nanoparticles for actuation and fully covered porous titania nanostructures as affinity coating. The multifunctional graphene composites can realize the selective capture and convenient magnetic separation of target phosphopeptides by taking advantage of the decorated magnetic nanoparticles, highly pure and well crystallized affinity coating, and unique porous structure. The affinity graphene composites can realize selective capture and rapid separation of low-abundant phosphopeptides from complex biological samples.
In another study, we prepared mesoporous and hollow carbon microspheres embedded with magnetic nanoparticles via a facile self-sacrificial method for rapid capture of low-abundant peptides from complex biological samples. The effectiveness of these affinity microspheres for capture of low-concentration peptides was evaluated by standard peptides, complex protein digests, and real biological samples. These multifunctional hollow carbon microspheres
can realize rapid capture and convenient separation of low-concentration peptides. They were validated to have better performance than magnetic mesoporous silica and commercial peptide-enrichment products. In addition, they can be easily recycled and present excellent reusability.
References:
-
Facile synthesis of magnetic mesoporous hollow carbon microspheres for rapid capture of low-concentration peptides, G. Cheng, M.-D. Zhou, S.-Y. Zheng, ACS Applied Materials & Interfaces, 6, 12719-12728, 2014. [Link]
-
Preparation of magnetic graphene composites with hierarchical structure for selective capture of phosphopeptides, G. Cheng, X. Yu, M. Zhou, and S.-Y. Zheng, J. Mater. Chem. B, 2, 4711-4719, 2014. [Link]
-
Graphene-Templated Synthesis of Magnetic Metal Organic Framework Nanocomposites for Selective Enrichment of Biomolecules, G. Cheng, Z. G. Wang, S. Denagamage, and S.-Y. Zheng, ACS Appl Mater Interfaces, 8, 10234-10242, 2016. [Link]
Nanomaterials for ultrafast protein digestion
We designed a magnetic enzyme nanosystem have by a polydopamine (PDA)-modification strategy (Figure a). The magnetic enzyme nanosystem has well defined core-shell structure and a relatively high saturation magnetization. The magnetic enzyme system can realize rapid, efficient and reusable tryptic digestion of proteins by taking advantage of its magnetic core and biofunctional shell. The magnetic enzyme nanosystem can digest the proteins in 30 minutes, and the results are comparable to conventional 12 hours in-solution digestion. The material can be reused several times, and has excellent stability for storage.
We further implement a similar strategy within a novel microfluidic protein digestion system with nanostructured and bioactive inner surface by an easy biomimetic covalent self-assembly strategy (Figure b). The microfluidic digestion system can realize rapid and effective proteolysis in 2 minutes, which is dramatically faster than the conventional overnight digestion methods by at least two orders of magnitude. Because of this advancement and the advantages of microfluidic chips, it is expected that this work would contribute to rapid online digestion in future high-throughput proteomics. Remote and noninvasive modulation of protein activity is essential for applications in biotechnology and medicine. In the 3rd example (Figure c), a near-infrared (NIR) light responsive graphene-silica-trypsin nanoreactor is developed for modulating the bioactivity of trypsin molecules. Biomolecules are spatially confined and protected in the rationally designed compartment architecture, which not only reduces the possible interference but also boosts the bioreaction efficiency. Upon NIR irradiation, the photothermal effect of the nanoreactor enables the ultrafast in situ heating for remote activation and tuning of the bioactivity. We apply the GST nanoreactor for remote and ultrafast proteolysis of proteins, which remarkably enhance the proteolysis efficiency and reduces the bioreaction time from the overnight of using free trypsin to seconds.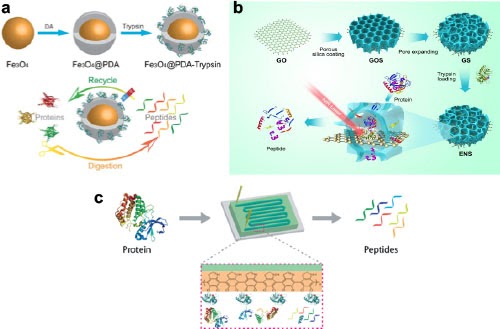
References:
-
Construction of a high-performance magnetic enzyme nanosystem for rapid tryptic digestion, G. Cheng and S.-Y. Zheng, Scientific Reports, 4: 6947, 1-10, doi:10.1038/srep06947, 2014. [pdf]
-
Nanostructured microfluidic digestion system for rapid high-performance proteolysis, G. Cheng, S.-J. Hao, X. Yu and S.-Y. Zheng, Lab on Chip, 15, 650-654, doi:10.1039/C4LC01165A, 2015. [Link]
-
In situ caging of biomolecules in graphene hybrids for light modulated bioactivity, G. Cheng, X.-H. Han, S.-J. Hao, M. Nisic, and S.-Y. Zheng, ACS Applied Materials & Interfaces, 10, 3361-3371, 2018. [Link]