EV-camouflaged nanoparticles for protein delivery
The intracellular delivery of biofunctional enzymes or therapeutic proteins through systemic administration is of great importance in therapeutic intervention of various diseases. However, current strategies face substantial challenges owing to various biological barriers, including susceptibility to protein degradation and denaturation, poor cellular uptake, and low transduction efficiency into the cytosol. We developed a biomimetic nanoparticle platform for systemic and intracellular delivery of proteins. Through a biocompatible strategy, guest proteins are caged in the matrix of metal-organic frameworks (MOF) with high efficiency and high loading capacity. The nanoparticles were further enveloped with the extracellular vesicle (EV) membrane. In vitro and in vivo study manifests that the EV-like nanoparticles can not only protect proteins against protease digestion and evade the immune system clearance but also selectively target homotypic tumor sites, promote tumor cell uptake and autonomous release of the guest protein after internalization. Assisted by biomimetic nanoparticles, intracellular delivery of bioactive therapeutic protein gelonin significantly inhibits the tumor growth in vivo.
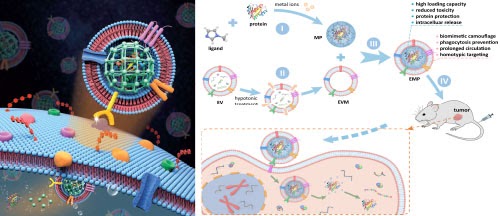
References:
-
Self-assembly of extracellular vesicle-like metal–organic framework nanoparticles for protection and intracellular delivery of biofunctional proteins, G. Cheng, W. Li, L. Ha, X. Han, S. Hao, Y. Wan, Z. Wang, F. Dong, X. Zou, Y. Mao, & S.-Y. Zheng, Journal of the American Chemical Society, 140, 7282-7291, 2018. (Feature as supplementary cover story) [Link] [PSU News Release]
Aptamer conjugated EVs for targeted drug delivery
The extracellular vesicles (EVs) have emerged as important disease biomarkers and therapeutic targets, but also have been utilized as novel drug delivery vehicles as EVs are dowered with small size, favorable surface protein composition, and membrane of the parental cells. However, clinical translations of EVs in targeted therapeutics are limited by their low yield and inadequate targeting effect. To produce large amount of EVs, cells grafted with lipidated targeting ligand (aptmer) were mechanically extruded to generate cancer cell targeting artificial EVs in ~1 hour. This rapid and economic approach could pave the way for clinical EV implementation in the future.
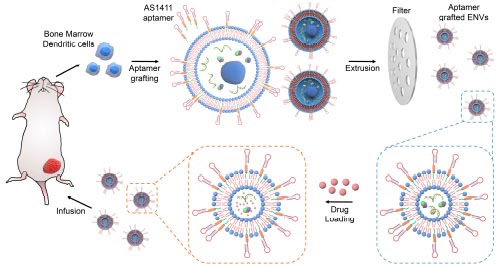
References:
-
Aptamer conjugated extracellular nanovesicles for in vivo targeted drug delivery, Y. Wan, L. Wang, C. Zhu, Q. Zheng, G. Wang, J. Tong, Y. Fang, Y.-Q. Xia, G. Cheng, X. He, and S.-Y. Zheng, Cancer Research, 78, 798-808, 2018. [Link]
Cellular Interactions
Nanomedicine-based drug delivery systems can have designed and sometimes unexpected interactions with cells. We studied using dopamine-derived polydopamine nanoparticles with a mitochondrial penetration molecule for mitochondria-targeted drug delivery to overcome drug resistance in cancer therapy (Figure a). In another case (Figure b), we discovered the preoccupation of endo-/lysosomes by the empty nanocarriers has a "saturation" effect mainly through changing the spatial distribution of the subsequently introduced nanocarriers. In the 3rd example (Figure c), we explored using mitochondria as a natural delivery system of carbon quantum dots for in vivo imaging and administration of the anticancer drug doxorubicin.
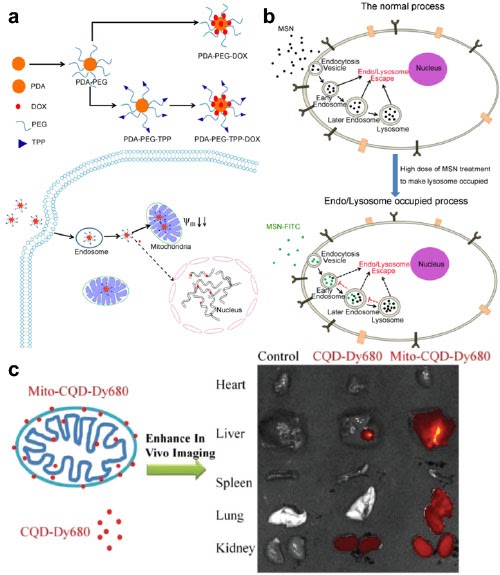
References:
-
Mitochondria-Targeting Polydopamine Nanoparticles to Deliver Doxorubicin for Overcoming Drug Resistance, W. Li, Z. Wang, S.-J. Hao, H.He, Y. Wan,C. Zhu, L. Sun, G. Cheng & S. -Y. Zheng, ACS Applied Materials & Interfaces, 9, 16793–16802, 2017.) [Link]
-
Pre-occupation of empty carriers decreases endo/lysosome escape and reduces the protein delivery efficiency of mesoporous silica nanoparticles, W. Li, L. Sun, Y. Xia, S.-J. Hao, G. Cheng, Z. Wang, Y. Wan, C. Zhu, H. He, and S.-Y. Zheng, ACS Applied Materials & Interfaces, 10, 5340-5347, 2018. [Link]
-
Mitochondria-based aircraft carrier enhances in vivo imaging of carbon quantum dots and delivery of anticancer drug, W.-Q. Li, Z. Wang, S. Hao, L. Sun, M. Nisic, G. Cheng, C. Zhu, Y. Wan, L. Ha, L. & S.-Y. Zheng, Nanoscale, 10, 3744-3752, 2018. [Link]