Research Team Improves Lithium Air Batteries for Electric Car Industry
By Emily Durham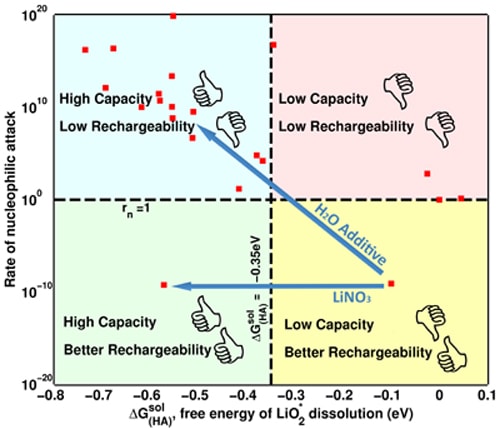 A research team from Carnegie Mellon University and the University of California, Berkeley, has found that blending together different types of salts in the electrolytes within lithium air batteries can increase the batteries' capacity while preserving their ability to be recharged.
A research team from Carnegie Mellon University and the University of California, Berkeley, has found that blending together different types of salts in the electrolytes within lithium air batteries can increase the batteries' capacity while preserving their ability to be recharged.
Lithium air batteries will soon be a strong competitor to lithium ion batteries, which currently dominate the battery-run electronics market. Lithium air batteries are especially promising for the electric car industry, as they allow electric car battery packs to be smaller and more lightweight and could hold more than twice as much energy as lithium ion batteries.
Batteries consist of one electrode on either side — an anode and a cathode — and an electrolyte between them. Lithium ion batteries' cathodes typically contain heavy metal ions like manganese, cobalt or iron, but in lithium air batteries, the much lighter oxygen in a sense acts as the cathode, creating a more efficient design. Electrolytes consist of a salt and a solvent to dissolve the salt.
“The electrolytes used in batteries are just like Gatorade electrolytes,” says Venkat Viswanathan, assistant professor of mechanical engineering at Carnegie Mellon. “Every electrolyte has a solvent and a salt. So if you take Gatorade, the solvent would be water and the salt would be something like sodium chloride, for instance. However, in a lithium air battery, the solvent is dimethoxyethane and the salt is something like lithium hexafluorophosphate.”
Earlier this year, a team led by Viswanathan and Assistant Professor Bryan McCloskey from the University of California, Berkeley, published a paper in Nature Chemistry, which showed that by adding just a little bit of water into the batteries' electrolyte mixture, the researchers were able to make the lithium air batteries last four to five times longer.
“However, adding water is not a perfect solution, because it comes at the cost of being able to recharge the battery,” cautioned Viswanathan, who explained that while water increased the battery capacity, it also catalyzed additional parasitic reactions, which prevented the batteries from being recharged.
Viswanathan, McCloskey and their colleagues — Mechanical Engineering Ph.D. Student Vikram Pande, Abhishek Khetan, a visiting Ph.D. student, and Colin Burke, a graduate student in McCloskey's lab — have just published a new paper in the Proceedings of the National Academy of Sciences, which addresses the rechargeability problem. The paper, titled "Enhancing Electrochemical Intermediate Solvation through Electrolyte Anion Selection to Increase Nonaqueous Li-O2 Battery Capacity,” explains that salts are the key — not solvents, as they'd previously thought.
“Our original idea was to add something to the solvent dimethoxyethane, and so we were intially exploring solvent additives. We found a fundamental problem with the solvent additives: the compromise to rechargeability. Then, McCloskey came up with the ingenious idea of changing the salt instead,” Viswanathan said. “Instead of using just one salt, we decided to blend salts together. We used two salts: lithium bis(trifluoromethane) sulfonimide and lithium nitrate.”
Pande adds, “By blending salts in the electrolyte solution, we increased the battery capacity by triggering the so-called solution process without compromising on rechargeability. Nitrate anion from the lithium nitrate salt does the trick by selectively dissolving previously insoluble products without facing the fundamental bottleneck produced by solvent additives.”
Though this research is an important advancement for lithium air batteries, the methods the researchers have developed will also be very impactful in other areas of battery research.
“This research is going to be very important for another big battery technology, lithium sulfur, and other competitors in the electric vehicle industry,” McCloskey said. “The whole idea of selectively making things soluble or insoluble is really important for most new battery ideas that are out there.”
Media Contact:
Tara Moore / 412-268-9673 / trmoore@andrew.cmu.edu