 |
|
 |
||||||
|
|
|
|||||||
|

Bone Tissue Engineering Center Carnegie Mellon and Pitt Center Works Toward Growing Bones With the nation aging and bones crumbling from use, a Pittsburgh multi-disciplinary team of researchers reaches for the not-so-elusive: lab-grown bones. By Janice Marchok Ryan When Jeffrey O. Hollinger graduated from the University of Maryland Dental School in 1973 and reported to the U.S. Army, he expected to serve his country as a staff dentist. Instead, his commanding officer said, "Captain, you're going to do bone." Hollinger spent the bulk of his 20-year Army career developing therapies for soldiers wounded on the battlefield. Now at Carnegie Mellon, he heads the Bone Tissue Engineering Center. Hollinger, his staff and his many collaborators envision a future in which health care providers can grow bone where none existed before. Patients like Emily Smith* motivate the new center's scientists. Emily has lived most of her 14 years wearing a hockey helmet to protect the half of her brain that has no skull bone protecting it. By 1993, then Col. Hollinger had completed a residency in oral and maxillofacial surgery and a Ph.D. in physiology. He retired from the military and joined the Oregon Health Sciences University faculty as a professor of surgery, anatomy and developmental biology in the School of Medicine. In addition to his research and teaching duties, Hollinger was also practicing as a maxillofacial surgeon. He was introduced to Emily when she was 9 years old.
When a bone breaks Another option that surgeons sometimes use is to graft bone from a cadaver. Although this option can also be effective, processing the bone is expensive, and the success rate is significantly lower. There are also concerns about transmitting diseases. Some man-made materials are already approved as bone substitutes; however, these materials can wear out, and they cannot grow or adapt to changes in the human body. "The goal of bone tissue engineering," says Hollinger, "is to apply innovative technologies to the knowledge that biologists have gained in past decades and improve current man-made materials to be more adaptable and versatile." In this case, scientific advances will grow out of old-fashioned communication. No single scientist has the breadth of knowledge necessary to grow bone. It's the collaboration—among engineers who can contribute their knowledge of manufacturing, physiologists who can share their knowledge of biological functions, and doctors who can provide input on patient needs and outcomes—that will solve this dilemma.
After he convinced others at Carnegie Mellon of the potential of this new field, Weiss recruited Jay Szem Calvert, a University of Pittsburgh Medical Center plastic surgeon, and Prashant Kumta, a Carnegie Mellon materials scientist. Within months, Carnegie Mellon had established an official Bone Tissue Engineering Initiative. Weiss, Calvert and Kumta continued to recruit a team with a wide breadth of expertise. By 2000, the initiative realized it needed someone with clinical experience and in-depth knowledge of bone and someone to orchestrate the diverse team. Hollinger came to Carnegie Mellon in July of 2000. Three Carnegie Mellon schools—Carnegie Institute of Technology, Mellon College of Science and the School of Computer Science and its Robotics Institute—pitched in space, funding and/or support. A few months later, the center opened for business.
Bones wear out with age Physiologist Phil Campbell, a research scientist in Carnegie Mellon's Institute for Complex Engineered Systems, says, "The longer you can keep moving around, the longer you're going to live. If you're stuck in a wheelchair or stuck in a bed—if you limit mobility—you start a downward spiral. Tissue engineering will offer solutions to keep people moving." With the number of people over 65 doubling, the nation can expect the $15 billion to $18 billion spent each year for the treatment of bone and joint conditions to more than double as well. Not only is there a humanitarian need for the technology, there is an equally profound economic impact brewing. The tissue engineers of Pittsburgh plan to offer solutions that will be significantly less expensive than those doctors have at their disposal now. Even more importantly, they plan to offer solutions that will have consistently predictable outcomes, unlike the less-than-optimal treatments in practice at present. "If a surgeon can purchase a lab-developed implant that can save the patient the risk, expense and recovery time of a second procedure and hospital stay," says Hollinger, "this represents a considerable cost savings. If the lab-developed implant can be engineered to include the precise composition of cells, molecules and matrix, surgical outcomes for patients will improve significantly." Engineering a strategy that can be used to create a human bone is an incredibly complex task, but Pittsburgh might just offer the perfect environment to undertake the mission. That's what Hollinger thought in 2000 when he turned down a prestigious offer to run Harvard's Craniofacial Tissue Engineering Center and instead chose to come to Carnegie Mellon. Pittsburgh has what no other city has, says Hollinger. "The decision not to go to Boston was easy," he adds. "The goal for the Carnegie Mellon tissue engineering effort was unambiguous. The people are genuine. Pittsburgh is a treasure chest lined with unlimited opportunities carried on the shoulders of an inspired team." That treasure chest includes the computer expertise of Carnegie Mellon and the medical expertise of the University of Pittsburgh Medical Center, not to mention a strong presence of scientists and engineers at both universities. Hollinger has engineered an impressive collaboration between the two universities and other entities in Pittsburgh.
When a bone heals "Mimicking this highly complex organization among hundreds of thousands of cells and hundreds of cell-signaling molecules is an incredible challenge," says Hollinger. "And the challenge is further complicated by the fact that the rate of healing and the ability to remodel a fractured bone vary for each person." A bone's ability to heal depends on a person's age, health, kind of fracture and the bone involved. A simple break in a normal 10-year-old, for example, might heal in two months but take four to five months to heal in a healthy 65-year-old. However, a 65-year-old diabetic with a long history of smoking will likely have significant delays and complications in the healing process. If this patient suffers a hip fracture, the healing will be hampered by limited blood flow to the fracture. Both nicotine and diabetes disrupt normal blood flow to the fracture and, thus, impede normal repair. If the patient becomes bedridden during the recovery, the immobility and restricted blood flow can combine to cause additional complications such as bedsores. Patients like this, who actually risk never regaining their mobility, are motivating the center's desire to develop effective tissue engineering treatments that will boost the local blood flow and reverse the impact of nicotine and diabetes.
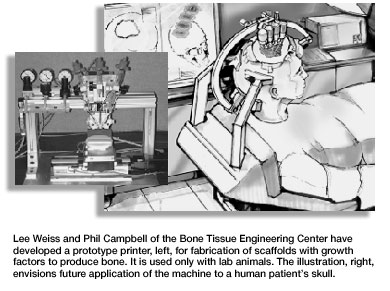
Many approaches explored; many questions raised Although researchers are exploring many approaches, most of the strategies involve some combination of signaling molecules, cells and/or a 3-D matrix or scaffold. For instance, the robotics engineer works with the materials scientist to manufacture a 3-D scaffold of the desired bone out of biodegradable materials. Simultaneously, they are working with the physiologists to carefully map out where the signaling molecules should be placed in the bone scaffold. They then seed the scaffold with cells, and the clinician implants the seeded scaffold into the patient. The goal is for cells to attach to the scaffold, multiply, then transform themselves from non-specific cells to cells exhibiting the bone-specific functions. The cells will then organize into normal, healthy bone as the implanted scaffold degrades. The huge number of variables involved makes the research slow and painstaking. If the area is small enough, you may not even need a scaffold. But if you do need a scaffold, what material should be used in the scaffold—a synthetic polymer, a ceramic, a native polymer or a composite? Where do you get the right cells to implant into the patient or seed the scaffold with—from the patient, another person or an animal? Do you seed the scaffold with the cells before surgery or during surgery? Do you even need cells, or will implanting just signaling molecules stimulate the person's existing cells to begin the bone formation process? And exactly where do you place the signaling molecules to get the correct message to the right spot at the appropriate time? Hollinger and his team have been able to get the recipe right in simple conditions—a relatively small bone area in an otherwise healthy patient. The challenge now is to scale that success up to be able to treat less-healthy patients and larger bone areas. This will be an evolutionary process that will take years to occur. Center scientists endure the disappointments of their trial-and-error experiments because they are confident that their ultimate goal will be reached. A new technology that Weiss and Campbell are developing has Hollinger excited. Introduced in March at the second annual Engineering Tissue Growth International Conference & Exposition in Pittsburgh, this technology is a printer of sorts that creates a 3-D scaffold and growth factors. Picture a pasta machine squeezing out a bone-shaped form. "Eventually," says Campbell, "this will be a mini-printer that is mounted onto a patient during surgery. The device will actually print the scaffold and growth factors directly into the patient." The center has accomplished much toward its goal of growing bones since July 2000. No scientific progress can occur without funding, and the center has secured over $4 million in grants and funding with another $4 million pending. Center scientists have six patents pending and have published seven papers with nine more accepted for publication.
But the hopes for the center are actually even broader than providing doctors with improved patient therapies and saving the U.S. government and taxpayers the burden of excessive health care costs. It is part of a larger initiative that combines Carnegie Mellon's computer expertise with the University of Pittsburgh's medical expertise. This partnership between two of the largest employers in Western Pennsylvania intends to turn Pittsburgh into the Silicon Valley of biomedical engineering. Known as the Pittsburgh Life Sciences Greenhouse, the effort aims to create 5,500 new biotech jobs. Both Pitt and Carnegie Mellon foresee beefed-up biomedical degree programs to train the biotech professionals of the future. Ultimately, center scientists envision giving the world a lot more than the ability to grow bone where none existed before. They also see a Pittsburgh revitalized by the tissue engineering industry, a Pittsburgh where the children of today become the researchers of tomorrow. And they see a future where no child has to wear a hockey helmet except to play the game.
> Back to the top > Back to Carnegie Mellon Magazine Home |
|||||||
|
Carnegie Mellon Home |
||||||||
 "It was a heartbreaking case," says Hollinger. The unprotected half of her head feels "like a ripe peach." Emily's doctors had previously tried to help her by performing a bone graft using bone from her hip. Unfortunately, the graft became infected. Not only did the infection destroy the grafted bone, it destroyed some of the normal skull around the graft site. Emily's mother desperately wanted her to be treated, so, in 1999, with special permission from the Food and Drug Administration and "compassionate use"
approval from the hospital review board, Hollinger and another surgeon operated on a half-dollar size area of Emily's skull. That graft was successful, so a second graft, about three by five inches, was performed when Emily was 11. That graft was also successful. Hollinger used BMP (bone morphogenetic protein) in the graft. This is the ingredient in bone that enables it to heal itself. These groundbreaking operations marked the first time a bone morphogenetic protein graft was used in a pediatric patient and the first time it was used to repair bone in the human skull.
"It was a heartbreaking case," says Hollinger. The unprotected half of her head feels "like a ripe peach." Emily's doctors had previously tried to help her by performing a bone graft using bone from her hip. Unfortunately, the graft became infected. Not only did the infection destroy the grafted bone, it destroyed some of the normal skull around the graft site. Emily's mother desperately wanted her to be treated, so, in 1999, with special permission from the Food and Drug Administration and "compassionate use"
approval from the hospital review board, Hollinger and another surgeon operated on a half-dollar size area of Emily's skull. That graft was successful, so a second graft, about three by five inches, was performed when Emily was 11. That graft was also successful. Hollinger used BMP (bone morphogenetic protein) in the graft. This is the ingredient in bone that enables it to heal itself. These groundbreaking operations marked the first time a bone morphogenetic protein graft was used in a pediatric patient and the first time it was used to repair bone in the human skull.
 Bringing the team together
Bringing the team together Phil Campbell of the Bone Tissue Engineering Center gets a head start on developing future biotech professionals by talking to local middle school and high school science classes as part of an outreach program coordinated by the Pittsburgh Tissue Engineering Initiative, a nonprofit organization founded in 1994 to foster tissue engineering in Pittsburgh.
Phil Campbell of the Bone Tissue Engineering Center gets a head start on developing future biotech professionals by talking to local middle school and high school science classes as part of an outreach program coordinated by the Pittsburgh Tissue Engineering Initiative, a nonprofit organization founded in 1994 to foster tissue engineering in Pittsburgh.