
Theoretical and Computational Biological Physics
Part of the Biological Physics Initiative
Carnegie Mellon University, Department of Physics
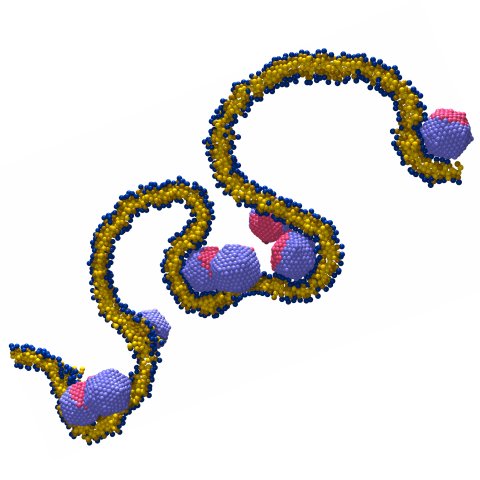
Curvy membranes make proteins attractive
Benedict J. Reynwar, Gregoria Illya, Vagelis A. Harmandaris, Martin M. Müller, Kurt Kremer, and Markus Deserno; Nature 447, 461–464 (2007).
The cells of our body are highly complex biochemical factories in which thousands of substances are created, processed and decomposed. To control this metabolism, all eukaryotic cells (such with a cell nucleus) possess various distinct organelles that are responsible for specialized tasks: Our genome is stored and read in the nucleus, proteins and lipids are synthesized in the endoplasmic reticulum, and the Golgi apparatus takes for instance the task of sorting proteins.
All these organelles are formed by lipid membranes. These are flexible double layers of lipid molecules, only five nanometers thin, which also make up the exterior envelope of each cell. In order to enable transport of material between the organelles - as well as into and out of the cell - they can change their shape. Particularly important is a process called "vesiculation" (see Fig. 1 on the right), during which a membrane bud forms that is later cut off the membrane. Proteins enclosed in the interior of such a "vesicle" can be transported to a different location inside the cell without getting mixed up along the way with other substances. How exactly all this happens is an important question in cell biology that is currently being studied with great intensity.
Since such membrane deformations cost energy, the cell drives them using special proteins. Today we know in many cases their identity. Yet, how they actually create a vesicle is much less understood. We have now used computer simulations to provide evidence for a physical mechanism that can lead to vesiculation. Remarkably, it is not necessary that the involved proteins interact with each other, for instance by mutual specific binding. Rather, proteins influence each other indirectly by the deformation of the lipid membrane which they cause by adhering to it
To create curved membrane structures, each protein has to bend the membrane a little bit. This local curvature spreads around a protein like a little "halo". When two proteins approach, the overlap of their halos may lead to an indirect interaction. One may think of the attraction between two balls lying on a tense rubber membrane. Usually this picture serves as an illustration of Einstein's theory of gravity by space-time curvature. And indeed these two seemingly different phenomena are formally closely related and can be described by similar mathematics.
Since almost two decades physicists have been on the track of membrane mediated interactions, yet the phenomenon remained confusing: on the one hand experiments documented attractions between membrane-bound objects. On the other hand all available theories indicated repulsion - at least if the membrane is curved uniformly. Since neither experiment nor theory are free of potential artifacts, the existence of curvature-mediated protein attractions remained elusive. Nevertheless, cell biologists started to be interested in the effect, as it promised a clear physical model of vesicle creation - provided it worked! The simulations performed in our group show that under suitable conditions (e.g. a minimum curvature imprint of each protein) the mechanism indeed leads to an attraction, and with enough proteins available to cooperative vesicle formation. For very strong curvatures the force can even be "measured" directly in the simulation (see Fig. 2 on the right).
The creation of transport vesicles is not just of fundamental importance for all life processes in cells, such as signal transduction or transport of nutrients. The involved mechanisms should also be at work in many other shape-determining tasks of membrane organelles. They furthermore play a role in the interaction of cell membranes with other objects, such as viruses or drugs. Since all this happens on sub-optical scales (about 100 nanometer), the experimental investigation of such events is a great challenge. Suitable computer simulations therefore support and complement available experimental techniques quite considerably.
The computing resources needed in this research project have been provided within the European project "DEISA" (Distributed European Infrastructure for Supercomputing Applications).

When proteins bind to cell membranes and, in doing so, bend them, they can attract each other indirectly due to the membrane deformations they cause. With enough proteins available this may lead to a membrane invagination. Using large-scale computer simulations we have for the first time verified this physical model for the initial steps of vesiculation in cells.
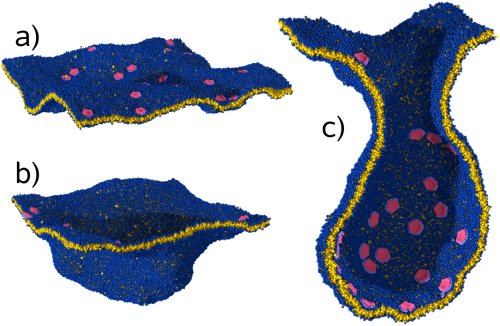
Figure 1: Vesiculation, as seen in a model simulation: a) pro-teins (red) adhere on a membrane (blue/yellow) and locally bend it; b) this triggers a growing invagination. In c) a cross-section through an almost complete vesicle is shown.
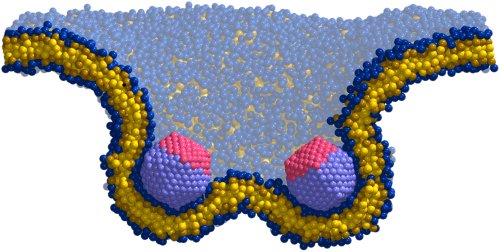
Figure 2: Cross-section of a membrane that is curved by two symmetrically adhering particles. In such a simulation the force between the particles can be "measured".

Looking for a postdoc, PhD position or under-graduate research project or similar? Have a look at our announcements!

Funding:


![]()