GenAI to Revolutionize Personalized Medicine
Tayur and Team Are Using Generative AI to Revolutionize Personalized Medicine
By Dr. Emily Barrow DeJeu and Dr. Sridhar Tayur
Dr. Sridhar Tayur of Carnegie Mellon’s Tepper School of Business, alongside Tepper PhD student H. Satyam Verma and Drs. Woody Zhu and Holly Wiberg from Carnegie Mellon’s Heinz College of Information Systems and Public Policy, is pioneering a bold new approach to personalized medicine by using generative AI to use limited patient data and refine drug dosage recommendations with expert input. Their method promises to overcome the data scarcity that limits precision medicine, achieving results comparable to massive real-world datasets with far less information.
The Problem: Dosing decisions are high-stakes, but personalization is nearly impossible with today’s data
Clinicians face a constant balancing act when determining how much of a drug to give a patient. The goal is to hit the therapeutic “sweet spot,” as the image on the left shows – enough to fight the disease effectively, but not so much that it causes intolerable side effects. But as the image on the right suggests, patients vary, and the optimal medication range for one might not work for another. This makes personalized dosing difficult.
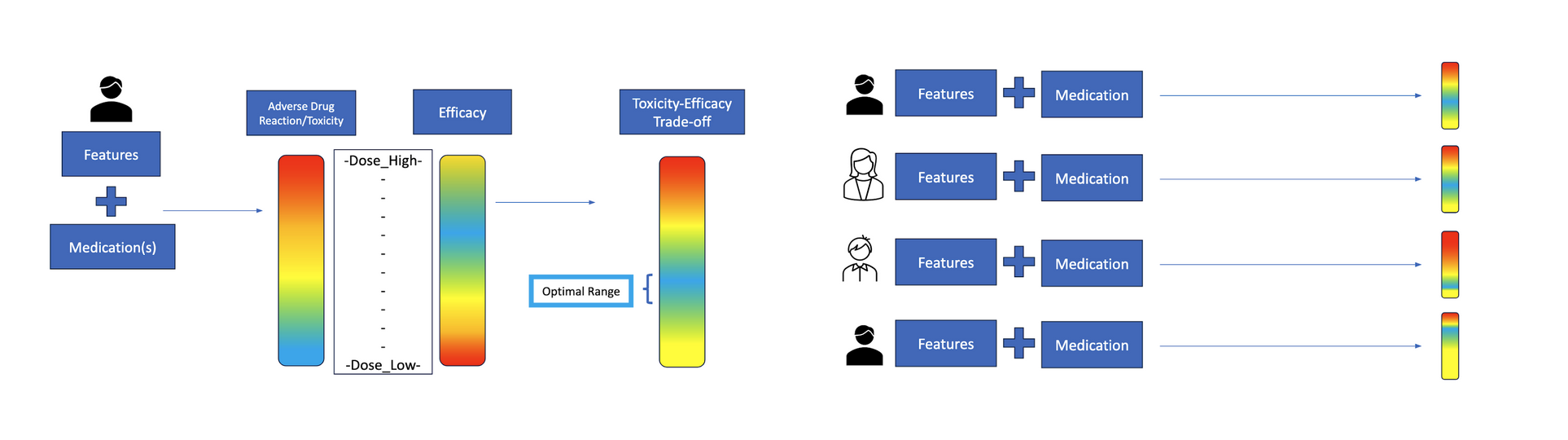
Right now, dosing is typically guided by broad protocols and physician intuition. A patient starts treatment, clinicians observe how they respond, and adjustments are made – sometimes after harmful side effects appear. This trial-and-error method can result in under-treatment that allows disease to progress or over-treatment that degrades quality of life. In cancer care, pain management, and other high-stakes settings, these tradeoffs can have life-altering consequences.
In theory, machine learning could offer a better way: algorithms that learn from data to recommend dosages tailored to a person’s unique biology. But in practice, this promise is blocked by a massive data gap, as the images below show. Because people differ so much—in genetics, comorbidities, lifestyles, and environments—the number of patient records needed to make truly personalized predictions is prohibitively large. As Tayur explains, “The amount of data available is very limited given the heterogeneity of the patients. The balance that works for you is not the balance that works for me.”
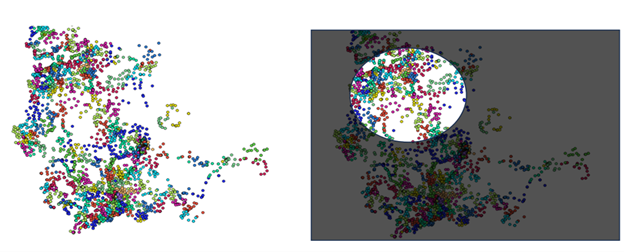
This leaves researchers and clinicians in a bind. The very concept of personalized medicine depends on individualized insights, but the data needed to generate those insights at scale doesn’t currently exist.
The Solution: Combining Synthetic Data with Expert Feedback to Improve Personalized Dosing
To overcome these data limitations, Tayur and his collaborators developed a novel approach they call GenEx, which combines generative AI for exploration with targeted expert feedback. The idea is simple but powerful: start with a small dataset of real patient records that are fully labeled, then use generative AI on other patient profiles that reflect the diversity and complexity of real-world populations to explore what experts may prefer among suggested dosage choices.
But the team didn’t stop there. Instead of treating all cases equally, they focused attention where it mattered most by identifying cases where model uncertainty was high or existing data was sparse. They then asked expert physicians to weigh in on these high-uncertainty scenarios. The physicians’ input helped refine the AI model, ultimately circumventing the need to fill in data gaps with real patient records. The image below illustrates this process:
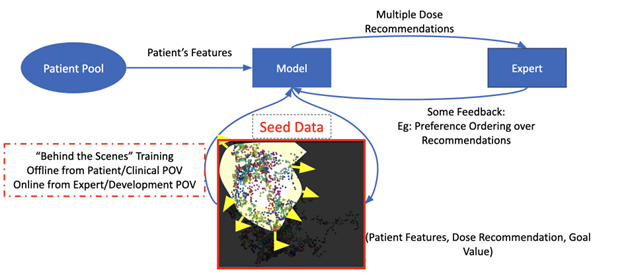
This hybrid framework—machine-generated data diversity plus human expertise—allowed the model to learn faster and more efficiently. In testing, Tayur’s team simulated a scenario where only 600 real patient records were available (compared to an original dataset of 25,000–40,000). By generating over 1,000 synthetic doses and incorporating expert feedback on just 100 or 200 of them, they were able to dramatically boost model performance.
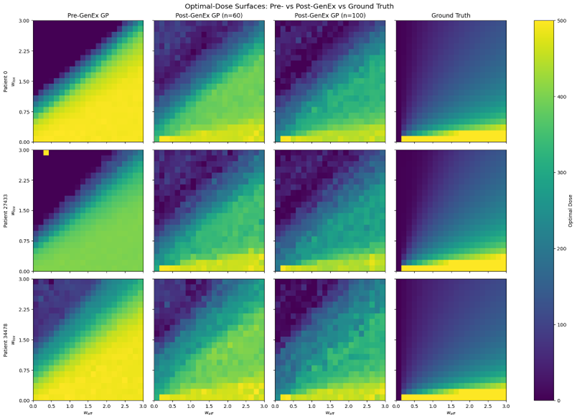
The result? Nearly the same accuracy as training on the full dataset at a fraction of the data cost, as the image below shows. As Tayur puts it, “We were able to replicate ground truth results with a fraction of the data—something that’s very promising for practical, real-world applications.”
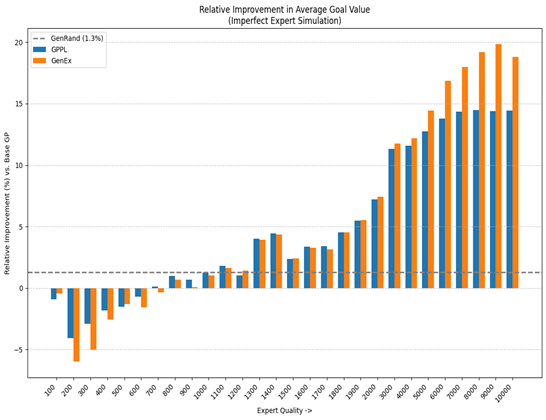
To further underscore the value of their hybrid method, Tayur and his colleagues compared three approaches: using only real-world data (dashed line), augmenting with expert feedback alone (blue curve), and combining generative data with expert input—the full GenEx method (orange curve). As shown in the chart below, our method improves over the benchmarks when the expert quality is higher, where we measure increased expert quality as the equivalent to having a higher number of data points. This visual evidence highlights the potential of generative AI not just for content creation, but as a powerful enabler of precision medicine.
What’s Next: From Pilot to Platform, and the Future of AI-Powered Precision Medicine
For companies exploring AI in healthcare, this research presents a scalable, cost-effective strategy for delivering personalized care in data-limited settings – precisely where traditional models fall short. By showing that generative AI, paired with expert insight, can deliver clinically viable results with minimal data, this team’s work sets the stage for a new class of AI tools that are not just powerful but practical and deployable. The potential extends well beyond dosing, opening the door to faster, more effective personalization across medicine’s most complex challenges.
