|
|
|||||
|
|
Scientists Develop Technology That Uses MRI To Visualize Gene Expression in Living Animals
"For 20 years it has been the chemist's job to develop agents that can be used to enhance MRI contrast," said Eric Ahrens, assistant professor of biological sciences in the Mellon College of Science at Carnegie Mellon. "Now, with our approach, we have put this job into the hands of the molecular biologist. Using off-the-shelf molecular biology tools we can now enable living cells to change their MRI contrast via genetic instructions."
"The new imaging method is a platform technology that can be adapted for many tissue types and for a range of preclinical uses in conjunction with emerging molecular therapeutic strategies," Ahrens said.
Ahrens' new approach uses magnetic resonance imaging (MRI) to monitor gene expression in real-time. Because MRI images deep tissues non-invasively and at high resolution, investigators don't need to sacrifice animals and perform laborious and costly analysis.
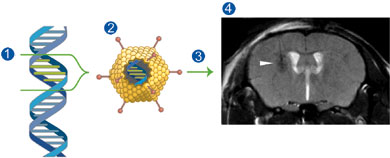
To trigger living cells into producing their own contrast agent, Ahrens gave them a gene that produces a form of ferritin, a protein that normally stores iron in a non-toxic form. This metalloprotein acts like a nano-magnet and a potent MRI "reporter."
A typical MRI scan detects and analyzes signals given off by hydrogen protons in water molecules after they are exposed to a magnetic field and radiofrequency pulses. These signals are then converted into an image. Ahrens' new MRI reporter alters the magnetic field in its proximity, causing nearby protons to give off a distinctly different signal. The resulting image reveals dark areas that indicate the presence of the MRI reporter.
"Our technology is adaptable to monitor gene expression in many tissue types. You could link this MRI reporter gene to any other gene of interest, including therapeutic genes for diseases like cancer and arthritis, to detect where and when they are being expressed," Ahrens said.
Existing methods used to image gene expression have limitations, according to Ahrens. Some methods cannot be used in living subjects, fail to image cells deep inside the body or don't provide highresolution images. Other approaches using MRI are not practical for a wide range of applications.
Ahrens and his colleagues constructed a gene carrier, or vector, that contained a gene for the MRI reporter. They used a widely studied vector called a replication-defective adenovirus that readily enters cells but doesn't reproduce itself. Ahrens injected the vector carrying the MRI reporter gene into brains of living mice and imaged the MRI reporter expression periodically for over a month in the same cohort of animals. The research showed no overt toxicity in the mouse brain from the MRI reporter.
Ahrens consulted on aspects of the research with William Goins, a research assistant professor at the University of Pittsburgh. The work was funded by the Pittsburgh Life Sciences Greenhouse and the National Institute of Biomedical Imaging and Bioengineering.
Ahrens is a member of the Pittsburgh NMR Center for Biomedical Research, a joint endeavor sponsored by Carnegie Mellon University and the University of Pittsburgh. Established in 1986 and funded continuously since 1988 by the National Institutes of Health, the Pittsburgh NMR Center is dedicated to advancing molecular, cellular and functional imaging in animals.
Amy Pavlak |
|||
|
Carnegie Mellon Home |
|||||