Microstructured Electrode Scaffold (MES)
In order to improve fuel cell and battery performance, we need to better understand the way charge (e.g. protons, electrons) and reacting species (e.g. oxygen) transport through the thickness of their electrodes. Why? Through-thickness (or through-plane) transport is one of the major sources of performance loss in these devices.
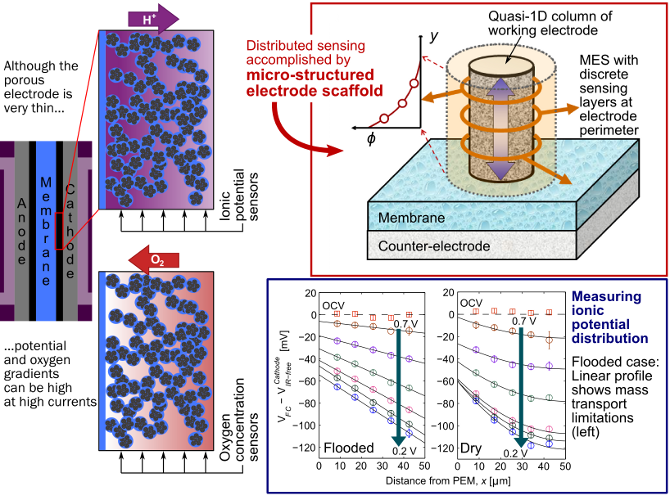
The electrode (sometimes called the catalyst layer) of a polymer electrolyte fuel cell is porous and very thin - depending on the type, it can be 5 to 50 µm. During reaction on the cathode side, the transport of protons and oxygen through this layer's winding pathways is critical to making the best use of the costly platinum catalyst spread throughout. However, in spite of its thinness, at high currents there can still be significant gradients in both the oxygen concentration (from mass transport) and the ionic potential (from proton transport) through the electrode.
With the goal of designing electrodes that have better transport properties, we first need to understand the different barriers oxygen and protons face when moving through the electrode. To that end, we employ the micro-structured electrode scaffold (MES).
Made using fairly simple thin-film methods, the MES is a substrate in which we can build a quasi-1D column of working fuel cell electrode (we primarily study the cathode). The MES brings sensing layers into contact with the side of the cathode, allowing us to measure e.g. the ionic potential at discrete points through the electrode. With several measurements of ionic potential through the thickness of the cathode, we can learn about proton transport in the layer. With a different build of MES, we can measure oxygen concentration and characterize oxygen transport in the cathode.
For more information, check out this brief presentation on MES diagnostics (PDF) or the following items:
- Journal paper: K. C. Hess, W. K. Epting, S. Litster, “Spatially Resolved, In Situ Potential Measurements through Porous Electrodes As Applied to Fuel Cells,” Analytical Chemistry, 83, pp. 9492-9498 (2011). [PDF] [News]
- Conference paper: W.K. Epting, K.C. Hess, and S. Litster, “In-Situ Measurement of Oxygen Partial Pressure in a Cathode Catalyst Layer,” Transactions of the Electrochemical Society, Las Vegas, NV, Oct. 10-15, 2010. [Paper on web]
- Conference paper: K.C. Hess, W.K. Epting, S.-C. Yu, and S. Litster, “In Situ Through-Plane Measurements of Ionic Potential in a PEMFC Catalyst Layer,” Transactions of the Electrochemical Society, accepted, Las Vegas, NV, Oc.t 10-15, 2010. [Paper on web]